CONCEPT14.2 Changes in Gene Expression Underlie Cell Fate Determination and Differentiation
Virtually every cell of an individual organism contains a complete copy of the organism’s genome. Each cell, however, expresses only a subset of these DNA sequences. For example, certain cells in hair follicles produce keratin, the protein that makes up hair, whereas other cell types in the body do not. In Chapter 5 we discussed cell-signaling pathways, many of which result in changes in gene expression. In Chapter 11 we described several mechanisms by which cells control gene expression—by controlling transcription and translation, and by making posttranslational protein modifications. As we mentioned in Concept 14.1, all four processes of development—determination, differentiation, morphogenesis, and growth—involve changes in gene expression, and these changes often result from signaling between cells. In this concept we focus on the processes of cell fate determination and differentiation.
The most fundamental decisions in development are generally controlled at the level of transcription. Genes that determine cell fate and trigger differentiation (often by regulating the expression of other genes on other chromosomes) usually encode transcription factors. In some cases, a single transcription factor can cause a cell to differentiate in a certain way. In other cases, multiple interactions between genes and proteins set off a sequence of transcriptional events that leads to differential gene expression. There are two ways in which cell fate can be determined:
280
- by the asymmetrical distribution of cytoplasmic factors inside a cell, so that its two progeny cells receive unequal amounts of the factors, or
- by the differential exposure of two cells to an external signal (an inducer).
Cell fates can be determined by cytoplasmic polarity
An early event in development is often the establishment of axes that relate to the body plan of the organism. For example, an embryo may develop a distinct “top” and “bottom” corresponding to what will become opposite ends of the mature organism; such a difference is called polarity. Many examples of polarity are observed as development proceeds. Our heads are distinct from our rear ends, and the distal (far from the center) ends of our arms and legs (wrists, ankles, fingers, toes) differ from the proximal (near) ends (shoulders and hips).
Polarity may develop early; even within the egg, the yolk and other factors are often distributed asymmetrically. During early development in animals, polarity is specified by an “animal pole” at the top of the zygote and a “vegetal pole” at the bottom. This polarity can lead to the determination of cell fates at a very early stage of development. For example, sea urchin embryos can be bisected at the eight-cell stage in two different ways:
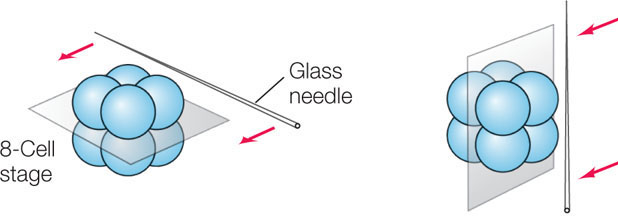
If the two halves (with four cells each) of these embryos are allowed to develop, the results are dramatically different for the two different cuts:
- For an embryo cut into a top half and a bottom half (left, above), the bottom half develops into a small sea urchin and the top half does not develop at all.
- For an embryo cut into two side halves (right, above), both halves develop into normal, though smaller, sea urchins.
These results indicate that the top and bottom halves of an eight-cell sea urchin embryo have already developed distinct fates. These observations led to the model of cytoplasmic segregation, which states that certain materials, called cytoplasmic determinants, are distributed unequally in the egg cytoplasm (FIGURE 14.7). During the early cell divisions of the embryo’s development, the progeny cells receive unequal amounts of these determinants. Cytoplasmic determinants include specific transcription factors that promote differential gene expression in the two daughter cells. They also include small regulatory RNAs and mRNAs, which also contribute to differential gene expression. What accounts for the unequal distribution of these determinants?
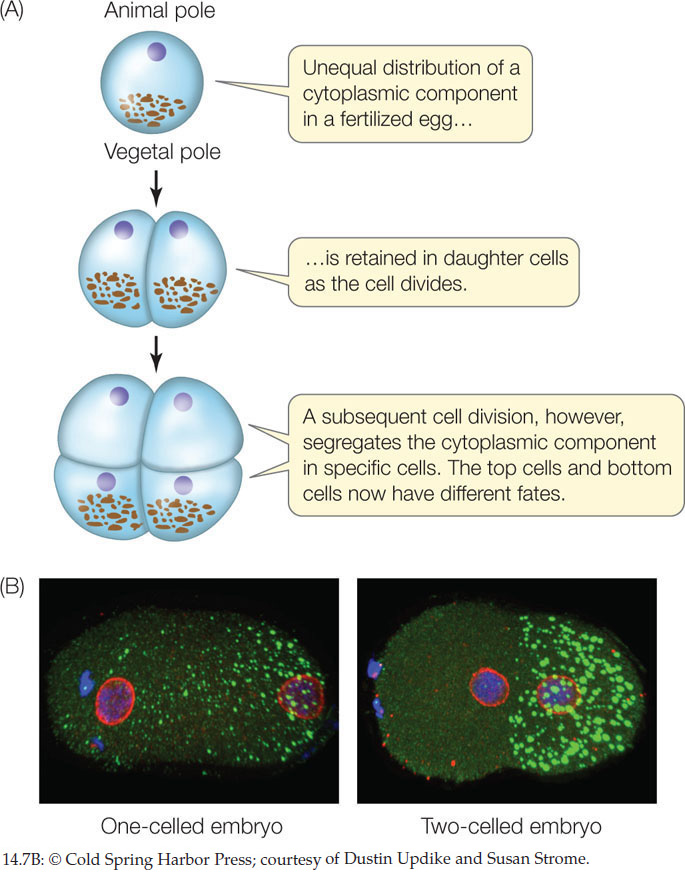
It turns out that the cytoskeleton contributes to the asymmetrical distribution of these determinants in the egg. Recall from Concept 4.4 that an important function of the microtubules and microfilaments in the cytoskeleton is to help move materials in the cell. Two properties allow these structures to accomplish this:
- Microtubules and microfilaments have polarity—they grow by adding subunits to the plus end.
- Cytoskeletal elements can bind specific proteins, which can be used in the transport of mRNA.
For example, in the sea urchin egg, a protein binds to both the growing (+) end of a microfilament and to an mRNA encoding a cytoplasmic determinant. As the microfilament grows toward one end of the cell, it carries the mRNA along with it. The asymmetrical distribution of the mRNA leads to asymmetrical distribution of the protein it encodes—a transcription factor.
Inducers passing from one cell to another can determine cell fates
The term “induction” has different meanings in different contexts. In biology it can be used broadly to refer to the initiation of, or cause of, a change or process. But in the context of cellular differentiation, it refers to the signaling events by which cells in a developing embryo communicate and influence one another’s developmental fate. Induction involves chemical signals called inducers and the signal transduction pathways that are triggered by these signals. Exposure to different amounts of inductive signals can lead to differences in gene expression among cells in a developing organism.
281
LINK
Signal transduction pathways are described in Concepts 5.5 and 5.6
The nematode worm Caenorhabditis elegans was one of the first model eukaryotic organisms to have its entire genome sequenced (see Concept 12.3). This worm develops from fertilized egg to larva in only about 8 hours and reaches the adult stage in just 3.5 days. The process is easily observed using a low-magnification dissecting microscope because the body covering is transparent (FIGURE 14.8A). To illustrate the principles of induction, we focus here on the development of one part of the C. elegans body: the vulva (FIGURE 14.8B).
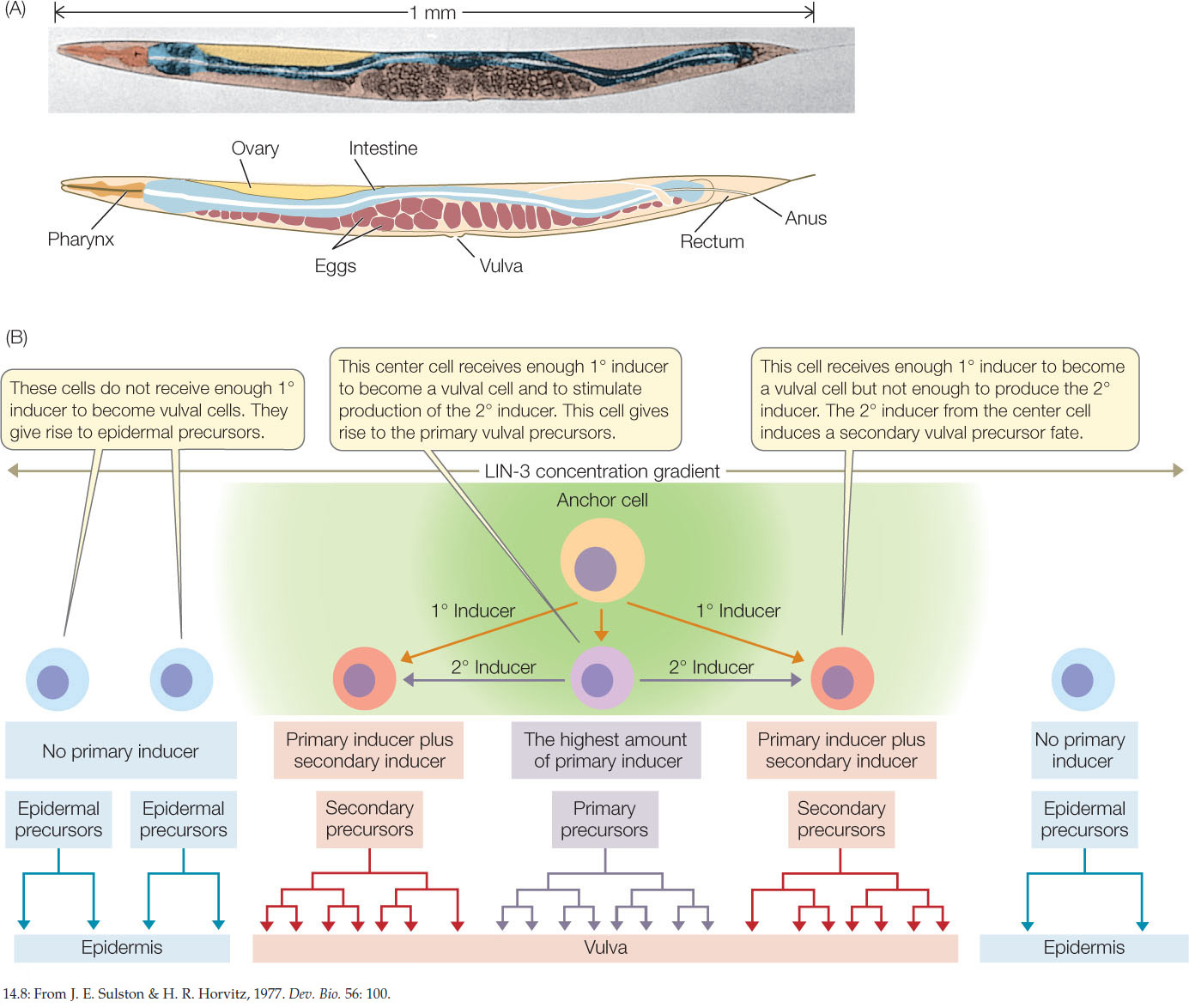
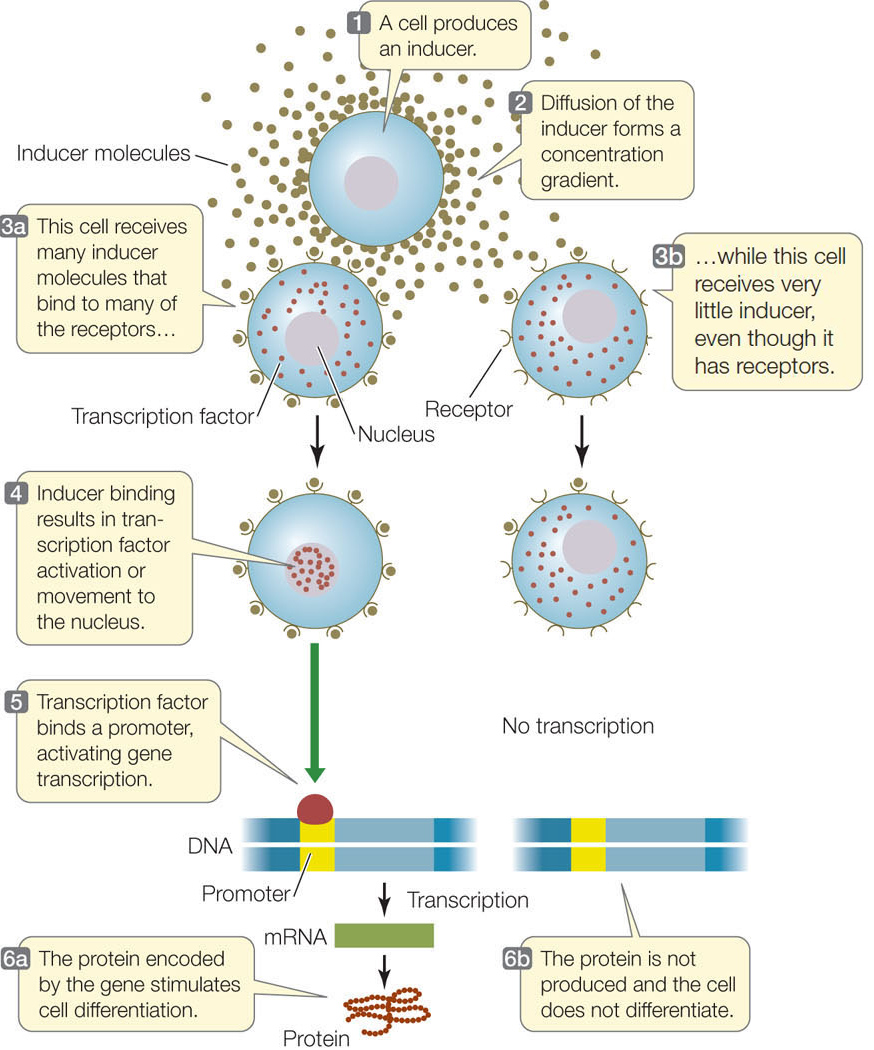
The adult nematode is hermaphroditic, containing both male and female reproductive organs. It lays eggs through a pore called the vulva on its ventral (lower) surface. During development, a single cell, called the anchor cell, induces the vulva to form from six cells on the worm’s ventral surface. In this case there are two molecular signals, the primary inducer and the secondary inducer. Each of the six ventral cells has three possible fates: it may become a primary vulval precursor cell, a secondary vulval precursor cell, or simply become part of the worm’s skin—an epidermal cell. You can follow the sequence of events in Figure 14.8B. The concentration of the primary inducer, LIN-3, is key: the anchor cell produces LIN-3, which diffuses out of the cell and forms a concentration gradient with respect to adjacent cells. The three cells that are closest to the anchor cell receive the most LIN-3 and become vulval precursor cells; cells slightly farther from the anchor cell receive less LIN-3 and become epidermal cells. A second induction event results in the two classes of vulval precursor cells: primary and secondary. Induction involves the activation or inactivation of specific sets of genes through signal transduction cascades in the responding cells (FIGURE 14.9).
282
This example from nematode development illustrates an important observation: much of development is controlled by molecular switches that allow a cell to proceed down one of two alternative paths. One challenge for developmental biologists is to find these switches and determine how they work. The primary inducer, LIN-3, released by the C. elegans anchor cell, is a growth factor similar in gene and protein sequence to a vertebrate growth factor called EGF (epidermal growth factor). LIN-3 binds to a receptor on the surfaces of vulval precursor cells, setting in motion a signal transduction cascade that results in increased transcription of the genes involved in the differentiation of vulval cells.
Differential gene transcription is a hallmark of cell differentiation
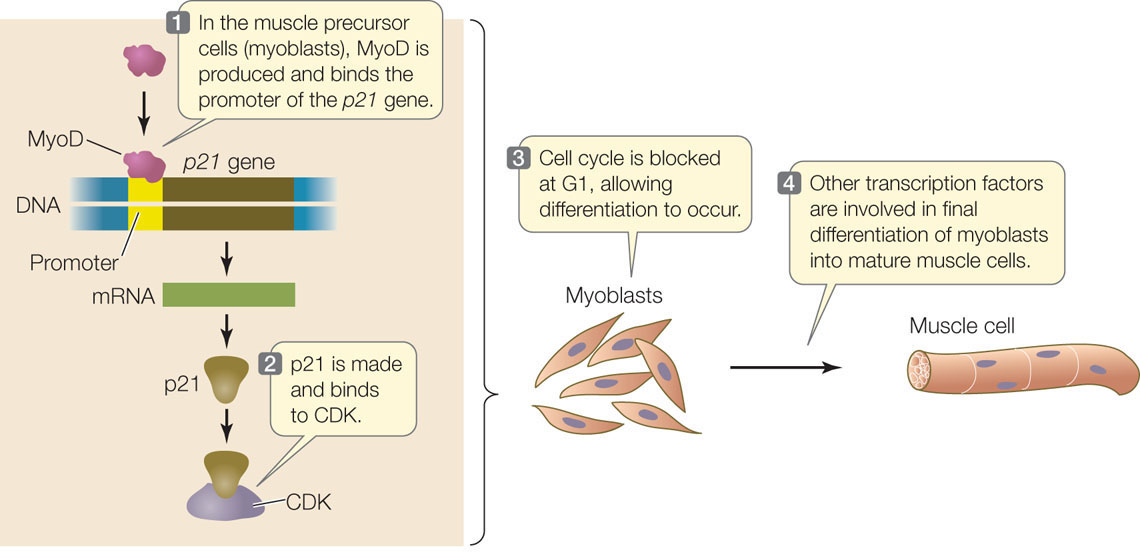
An important mechanism by which cells differentiate into specific cell types, with specific functions, is differential gene transcription. One well-studied example of cell differentiation is the conversion of undifferentiated muscle precursor cells (myoblasts) into the cells that make up muscle fibers (FIGURE 14.10). A key event in the commitment of these cells to become muscle is that they stop dividing. Indeed, in many parts of the embryo, cell division and cell differentiation are mutually exclusive. Cell signaling activates the gene for a transcription factor called MyoD (myoblast-determining gene). MyoD activates the gene for p21, an inhibitor of cyclin-dependent kinases (CDKs) that normally stimulate the cell cycle at G1 (see Figure 7.10). Expression of the p21 gene causes the cell cycle to stop, and other transcription factors then enter the picture so that myoblasts can differentiate into muscle cells.
CHECKpointCONCEPT14.2
- What would be the effect on the embryo of injecting an inhibitor of microtubule polymerization into a fertilized sea urchin egg?
- Compare the internal and external stimuli that lead to differential gene expression in embryonic cells.
- What would be the consequences of a homozygous deletion mutation of lin-3, the gene that encodes LIN-3?
283
Cytoplasmic polarity and inducers affect the expression of genes that determine cell fates. We will now look in more detail at how spatial differences in gene expression affect cell fate determination and the formation of tissues and organs.