CONCEPT26.4 Photoreceptors Initiate Developmental Responses to Light
Plants are completely dependent on sunlight for energy, so it is not surprising that they have multiple ways to sense light and respond appropriately. A number of physiological and developmental events in plants are controlled by light, a process called photomorphogenesis. Plants respond to two aspects of light: its quality—that is, the wavelengths of light to which the plant is exposed; and its quantity—that is, the intensity and duration of light exposure.
568
Chapter 6 described how chlorophyll and other photosynthetic pigments absorb light of certain wavelengths (see Figure 6.17) and how plants use light energy to synthesize carbohydrates. Here we consider how light affects plant development. Light influences seed germination, phototropism, shoot elongation, initiation of flowering, and many other important aspects of plant development. Several photoreceptors take part in these processes.
Phototropin, cryptochromes, and zeaxanthin are blue-light receptors
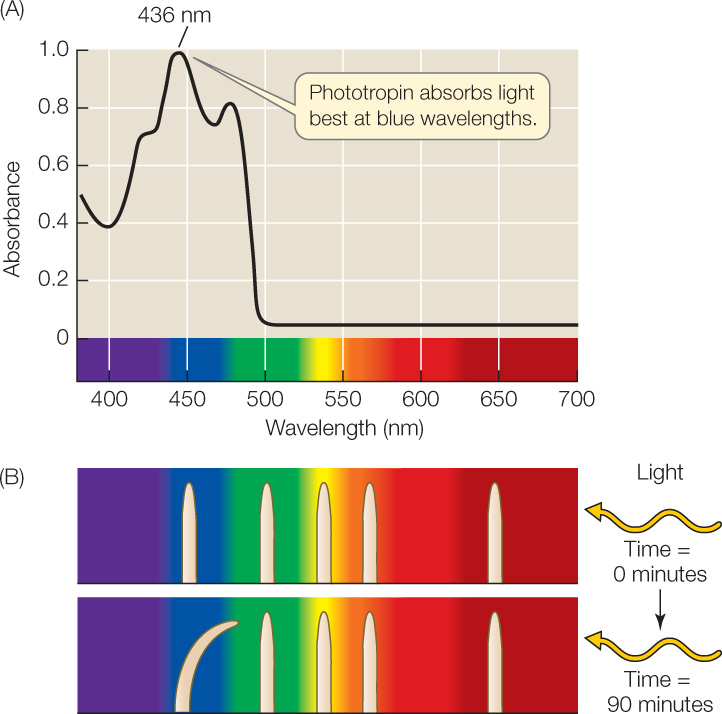
An action spectrum for the phototropic response of a coleoptile (FIGURE 26.10) shows that blue light at a wavelength of 436 nm is the most effective in stimulating that response. To identify the blue-light-absorbing molecule involved, biologists have taken a genetic approach using the model plant Arabidopsis.
LINK
Action spectra are discussed in Concept 6.5
By obtaining blue-light-insensitive Arabidopsis mutant plants from a genetic screen, researchers were able to identify the gene for a blue-light receptor protein located in the cell membrane, called phototropin. This protein has a flavin mononucleotide associated with it that absorbs blue light at 436 nm. Light absorption leads to a change in the shape of the protein that exposes an active site for a protein kinase, which in turn initiates a signal transduction cascade that ultimately results in stimulation of cell expansion by auxin. Phototropin also participates with another type of blue-light receptor, the plastid pigment zeaxanthin, in the light-induced opening of stomata (see Figure 25.13).
A third class of blue-light receptors is the cryptochromes, proteins with yellow pigments that absorb blue and ultraviolet light. These pigments are located primarily in the plant cell nucleus and affect seedling development and flowering. The exact mechanism of cryptochrome action is not yet known. Strong blue light inhibits cell expansion through the action of cryptochromes, although the most rapid responses are mediated by phototropin.
Phytochrome senses red and far-red light
Plants also have responses that are specific to light in the red region of the spectrum. Here are two examples:
- Lettuce seeds spread on soil will germinate only in response to light. Even a brief flash of dim light will suffice. This response prevents the seeds from germinating when they are so deep in the soil that the seedlings would not be able to reach the light before their seed reserves ran out.
- Adult cocklebur plants flower when they are exposed to long nights. If there is even a brief flash of light in the middle of the night, they do not flower. This response enables plants to flower in the appropriate season.
The action spectra of these processes show that they are induced by red light (650–680 nm). This indicates that plants must have a photoreceptor that absorbs red light.
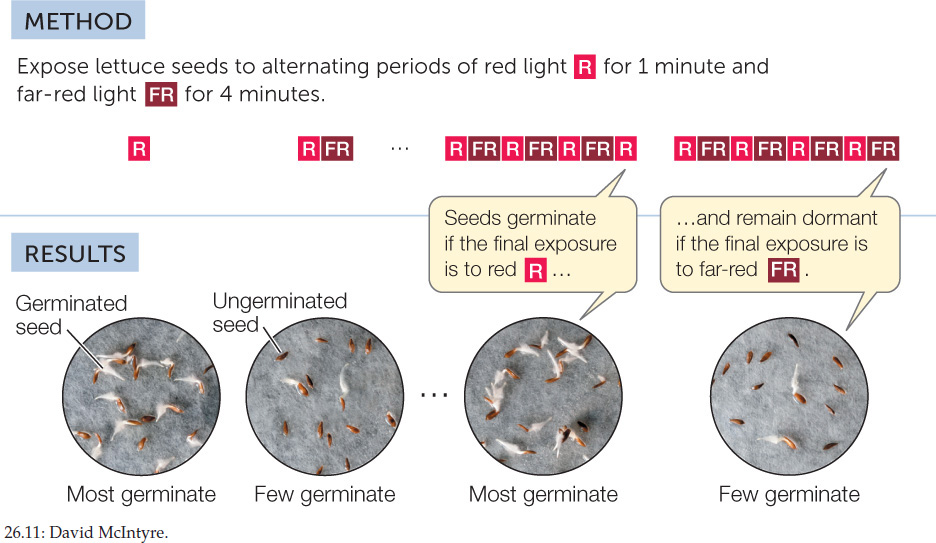
What is especially remarkable about these red light responses is that they are reversible by far-red light (710–740 nm). For example, if lettuce seeds are exposed to brief, alternating periods of red and far-red light in close succession, they respond only to the final exposure. If it is red, they germinate; if it is far-red, they remain dormant (FIGURE 26.11). This reversibility of the effects of red and far-red light regulates many other aspects of plant development, including flowering and shoot development.
Investigation
HYPOTHESIS
The effects of red and far-red light on lettuce seed germination are mutually reversible.
CONCLUSION
Red light and far-red light reverse each other’s effects.
ANALYZE THE DATA
Seven groups of 200 lettuce seeds each were incubated in water for 16 hours in the dark. One group was then exposed to white light for 1 minute. A second group (controls) remained in the dark. Five other groups were exposed to red (R) and/or far-red (FR) light. All the seeds were then returned to darkness for 2 more days. The table shows the number of seeds that germinated in each group.
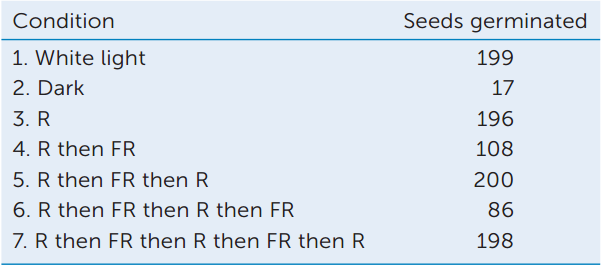
- Calculate the percentage of seeds that germinated in each case.
- What can you conclude about the photoreceptors involved?
aH. Borthwick et al. 1952. Proceedings of the National Academy of Sciences USA 38: 662–666.
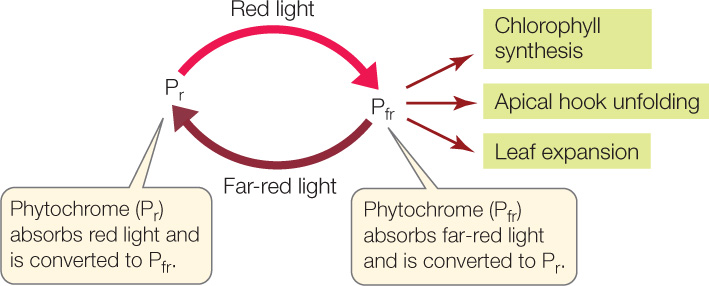
The basis for the effects of red and far-red light is a bluish photoreceptor in the cytosol of plants called phytochrome. Phytochrome exists in two interconvertible isoforms, or states. The molecule undergoes a change in shape when it absorbs light at particular wavelengths. The default or “ground” state, which absorbs principally red light, is called Pr. When Pr absorbs a photon of red light, it is converted into the other isoform, Pfr. The Pfr isoform preferentially absorbs far-red light; when it does so, it is converted back into Pr:
569
Pfr, not Pr, is the active isoform of phytochrome—the form that triggers important biological processes in various plants. These processes include chlorophyll synthesis, apical hook unfolding, and leaf expansion.
The ratio of red to far-red light determines whether a phytochrome-mediated response will occur. For example, in full daylight the ratio is about 1.2:1; because there is more red than far-red light, the Pfr isoform predominates. But for a plant growing in the shade of other plants, the ratio may be as low as 0.13:1, and most phytochrome is in the Pr isoform. The low ratio of red to far-red light in the shade results from absorption of red light by chlorophyll in the leaves overhead, so less of the red light gets through to the plants below. Shade-intolerant species respond by stimulating cell expansion in the stem and thus growing taller to escape the shade (similar to the behavior of etiolated seedlings grown in the dark). Similarly, shade cast by other plants prevents germination of seeds that require red light to germinate (see Figure 26.11).
Phytochrome stimulates gene transcription
How does phytochrome—or more specifically, Pfr—work? Phytochrome is a cytoplasmic protein composed of two subunits (FIGURE 26.12). Each subunit contains a protein chain and a nonprotein pigment called a chromophore. In Arabidopsis, there is a gene family that encodes five slightly different phytochromes, each functioning in different photomorphogenic responses.
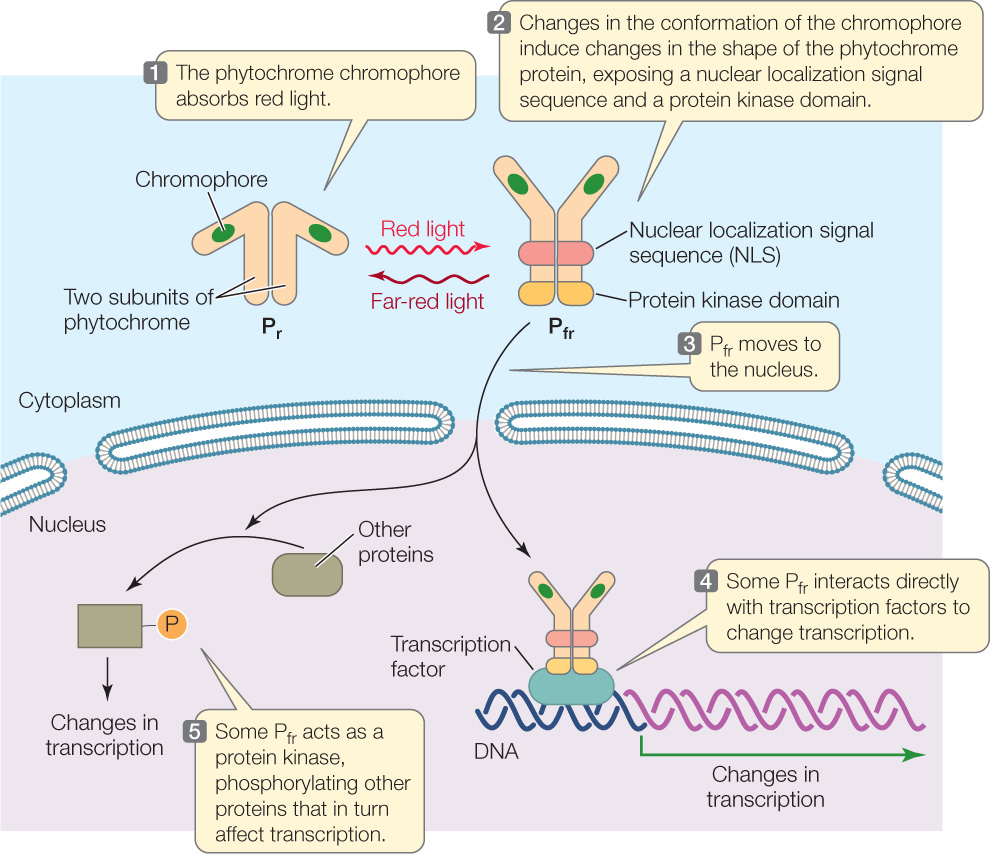
The transcription of genes involved in phytochrome responses is changed when Pr is converted into the Pfr isoform. When Pr absorbs red light, the chromophore changes shape, which leads to a change in the conformation of the phytochrome protein from the Pr isoform to the Pfr isoform. Conversion to the Pfr isoform exposes two important regions of the protein (see Figure 26.12), both of which affect transcriptional activity:
- Exposure of a nuclear localization signal sequence (see Figure 10.20) results in movement of Pfr from the cytosol to the nucleus. Once in the nucleus, Pfr binds to transcription factors and thereby stimulates expression of genes involved in photomorphogenesis.
- Exposure of a protein kinase domain causes the Pfr protein to phosphorylate itself and other proteins involved in red-light signal transduction. Those proteins change the activity of other transcription factors.
The effect of this activation of transcription factors is quite large: in Arabidopsis, phytochrome affects an amazing 2,500 genes (10 percent of the entire genome) by either increasing or decreasing their expression. Some of these genes are related to other hormones. For example, when Pfr is formed at the time of seed germination, genes for gibberellin synthesis are activated and genes for gibberellin breakdown are repressed. As a result, gibberellins accumulate and seed reserves are mobilized (see Figure 26.4).
570
Circadian rhythms are entrained by photoreceptors
The timing and duration of biological activities in living organisms are governed in all eukaryotes and some prokaryotes by what is commonly called a biological clock: an oscillator within cells that alternates back and forth between two states at roughly 12-hour intervals. The major outward manifestations of this clock are known as circadian rhythms (Latin circa, “about”; dies, “day”). In plants, circadian rhythms influence, for example, the opening (during the day) and closing (at night) of stomata in Arabidopsis and the elevation toward the sun (during the day) and lowering (at night) of leaves in bean plants.
LINK
For a description of the role of circadian rhythms in animal behavior, see Concept 40.4
The circadian rhythms of plants can be reset, or entrained, within limits, by light–dark cycles that change. Consider what happens when plants adapt to day length as the seasons change during the year. The action spectrum for plant entrainment indicates that phytochrome (and to a lesser extent, blue-light receptors) is very likely involved. At sundown, phytochrome is mostly in the active Pfr isoform. But as the night progresses, Pfr is gradually converted back into the inactive Pr isoform. By dawn, most phytochrome is in the Pr state, but as daylight begins, it is rapidly converted into Pfr. The switch to the Pfr isoform resets the plant’s biological clock. However long the night, the clock is still reset at dawn every day. Thus while the total period measured by the clock does not change, the clock adjusts to changes in day length over the course of the year.
CHECKpointCONCEPT26.4
- Trace the events that occur in the phytochrome-mediated germination response of lettuce seeds, from the reception of light to alteration in gene expression.
- Compare the wavelengths of light absorbed by chlorophyll (see Figure 6.17) and by phototropin (see Figure 26.10). How does the difference influence a plant’s responses to light when a plant grows beneath a forest canopy?
- How would you show that a physiological process in a plant is brought about by phytochrome?
What changes in their growth patterns made the new strains of cereal crops produced by the Green Revolution so successful?
ANSWER It is only recently that plant biologists have discovered why semi-dwarf wheat and rice have short stems (FIGURE 26.13). This knowledge has come from an understanding of the effects of various hormones on plant growth and development (Concept 26.1), the signal transduction pathways for response to these hormones (Concepts 26.2 and 26.3), and the biochemical pathways for the synthesis of these hormones. It turns out that the genes involved in wheat and in rice are different.

571
In semi-dwarf wheat, the mutant allele that produces short plants is involved in signal transduction in response to gibberellin. The gene involved is called Rht (“reduced height”). The mutant allele was first seen in Japanese semi-dwarf varieties. It is dominant, so its phenotypes are readily seen. Norman Borlaug used conventional plant breeding to transfer the Rht mutant allele into wheat strains in Mexico. In addition to having short stature and not falling over when bearing a high grain yield, Rht mutant plants put a greater proportion of their photosynthate into making seeds than wild-type plants do. This higher “harvest index” is also a major reason why most wheat varieties today carry an Rht mutation.
A hint that Rht might be involved in hormone regulation came when semi-dwarf plants harboring Rht mutations were found to be insensitive to added gibberellin and did not grow to normal height when the hormone was applied. Rht encodes the transcriptional repressor in the gibberellin signal transduction pathway (see Figure 26.8). Mutations in semi-dwarf plants are in the region that binds the gibberellin receptor and that signals polyubiquitination and degradation in the proteasome. Thus in the mutant plants, the repressor is always bound to the transcription factor, inhibiting its activity and keeping the gibberellin response “off.” This mutation therefore results in shorter stature.
In semi-dwarf rice, the mutant allele that produces short plants is involved with gibberellin synthesis. The gene involved is called sd-1 (“semi-dwarf”). In contrast to semi-dwarf wheat, rice plants with mutations in sd-1 are sensitive to gibberellin—that is, the semi-dwarf phenotype can be corrected by supplying gibberellin. This observation indicates that the signal transduction machinery is intact in the mutant plants but that they lack the hormone. Indeed, sd-1 encodes an enzyme that catalyzes one of the last steps in gibberellin synthesis.