CONCEPT6.5 During Photosynthesis, Light Energy Is Converted to Chemical Energy
The energy released by catabolic pathways in almost all organisms, including animals, plants, and prokaryotes, ultimately comes from the sun. Photosynthesis (literally, “synthesis from light”) is an anabolic process by which the energy of sunlight is captured and used to convert carbon dioxide (CO2) and water (H2O) into carbohydrates (which we represent as a six-carbon sugar, C6H12O6) and oxygen gas (O2):
119
6 CO2 + 6 H2O → C6H12O6 + 6 O2
This equation shows a highly endergonic reaction. The net outcome is the reverse of the general equation for glucose catabolism that we discussed in Concept 6.2. Many of the molecular processes of photosynthesis are similar to those for glucose catabolism. For example, both processes involve redox reactions, electron transport, and chemiosmosis. However, the details of photosynthesis are quite different.
Photosynthesis involves two pathways (FIGURE 6.15):
- The light reactions convert light energy into chemical energy in the form of ATP and the reduced electron carrier NADPH. This molecule is similar to NADH (see Figure 6.4A) but with an additional phosphate group attached to the sugar of its adenosine.
- The carbon-fixation reactions do not use light directly, but instead use the ATP and NADPH made by the light reactions, along with CO2, to produce carbohydrates.
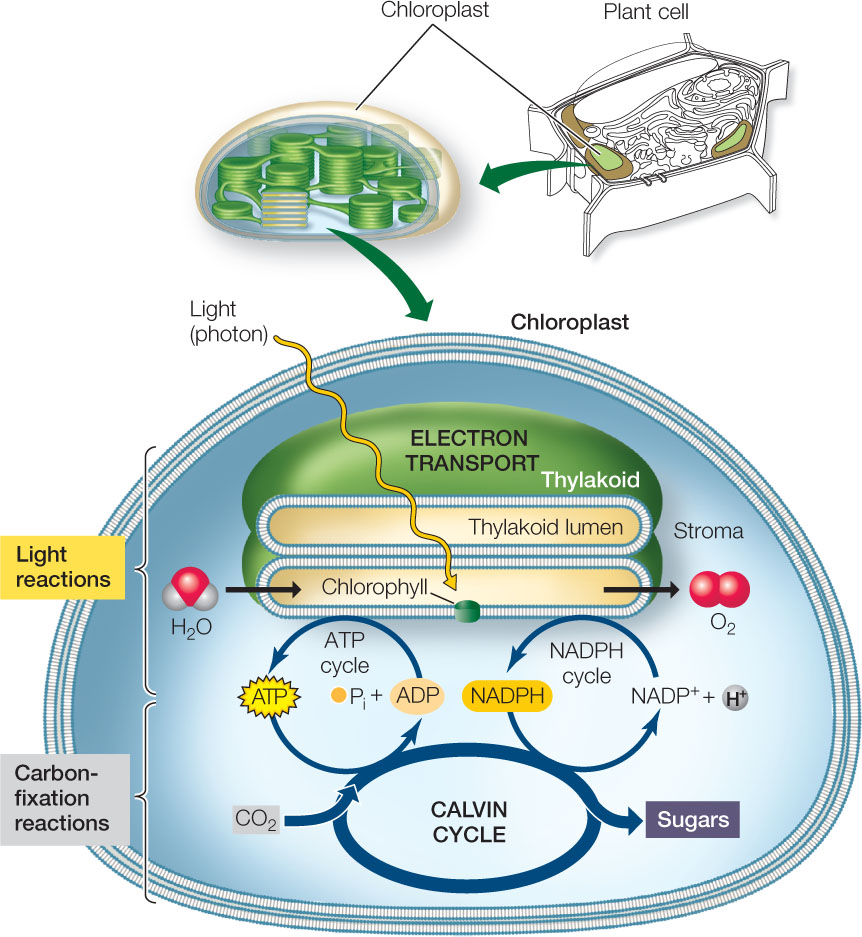
Both the light reactions and the carbon-fixation reactions stop in the dark because ATP synthesis and NADP+ reduction require light. In photosynthetic prokaryotes (e.g., cyanobacteria), the light reactions take place on internal membranes and the carbon-fixation reactions occur in the cytosol. In plants, which will be our focus here, both pathways proceed within the chloroplast, but they occur in different parts of that organelle (see Figure 6.15).
Light energy is absorbed by chlorophyll and other pigments
Light is a form of energy that can be converted to other forms, such as heat or chemical energy. It is helpful here to discuss light in terms of its photochemistry and photobiology.
Photochemistry
Light is a form of electromagnetic radiation. Electromagnetic radiation is propagated in waves, and the amount of energy in the radiation is inversely proportional to its wavelength—the shorter the wavelength, the greater the energy. The visible portion of the electromagnetic spectrum (FIGURE 6.16) encompasses a wide range of wavelengths and energy levels. In addition to traveling in waves, light also behaves as particles, packets of light energy called photons, which have no mass. In plants and other photosynthetic organisms, receptive molecules absorb photons in order to harvest their energy for biological processes. These receptive molecules absorb only specific wavelengths of light—photons with specific amounts of energy.
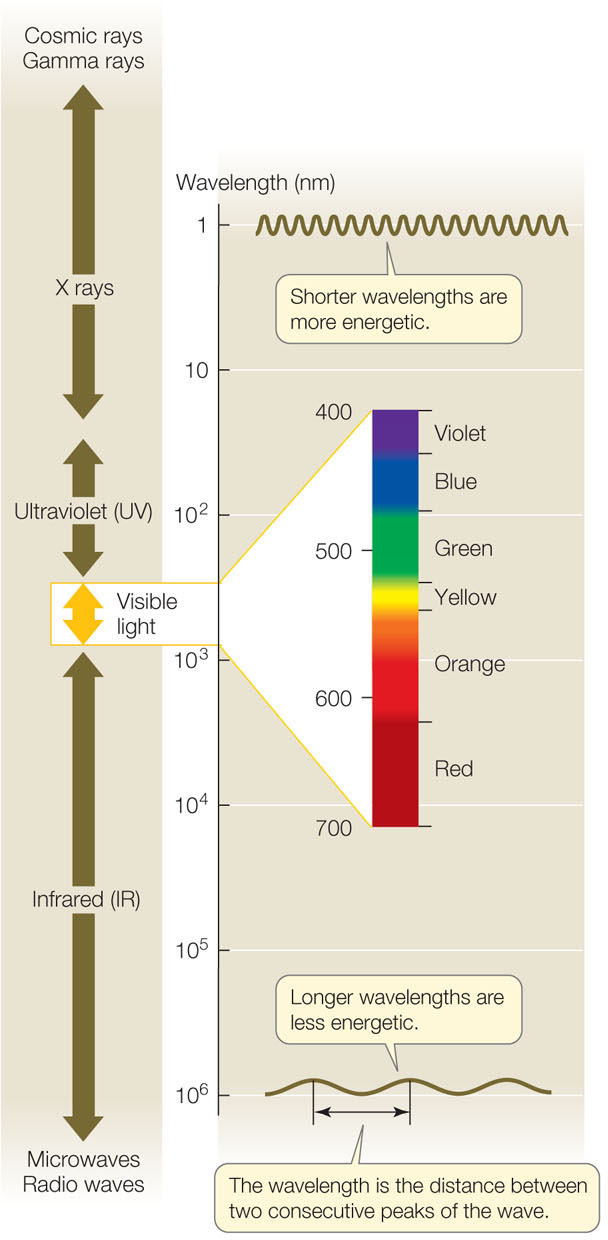
When a photon meets a molecule, one of three things can happen:
- The photon may bounce off the molecule—it may be scattered or reflected.
- The photon may pass through the molecule—it may be transmitted.
- The photon may be absorbed by the molecule, adding energy to the molecule.
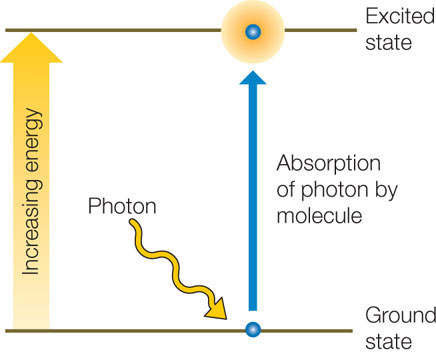
Neither of the first two outcomes causes any change in the molecule. However, in the case of absorption, the photon disappears and its energy is absorbed by the molecule. The photon’s energy cannot disappear, because according to the first law of thermodynamics, energy is neither created nor destroyed. When the molecule acquires the energy of the photon, it is raised from a ground state (with lower energy) to an excited state (with higher energy):
120
The difference in free energy between the molecule’s excited state and its ground state is approximately equal to the free energy of the absorbed photon (a small amount of energy is lost to entropy). The increase in energy boosts one of the electrons within the molecule into a shell farther from its nucleus; this electron is now held less firmly, making the molecule unstable and more chemically reactive.
Photobiology
Each type of molecule absorbs light at specific, characteristic wavelengths. Molecules that absorb wavelengths in the visible spectrum are called pigments.
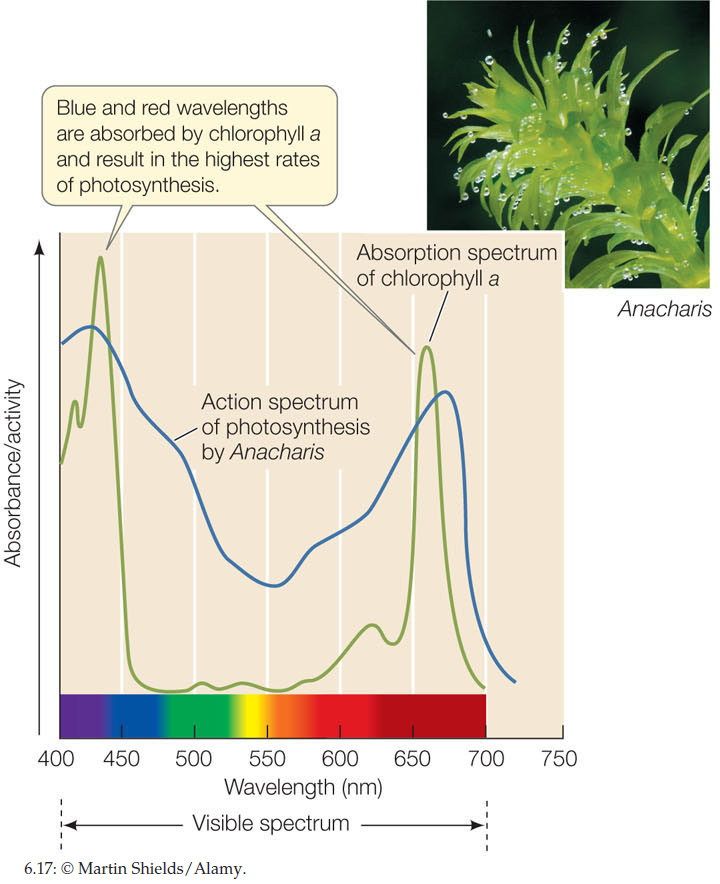
When a beam of white light (containing all the wavelengths of visible light) falls on a pigment, certain wavelengths are absorbed. The remaining wavelengths are scattered or transmitted and make the pigment appear to us as colored. For example, the pigment chlorophyll absorbs both blue and red light, and we see the remaining light, which is primarily green. If we plot light absorbed by a purified pigment against wavelength, the result is an absorption spectrum for that pigment.
In contrast to the absorption spectrum, an action spectrum is a plot of the biological activity of an organism against the wavelengths of light to which it is exposed. An action spectrum can be determined as follows:
- Place the organism (for example, a water plant with thin leaves) in a closed container.
- Expose it to light of a certain wavelength for a period of time.
- Measure the rate of photosynthesis by the amount of O2 released.
- Repeat with light of other wavelengths.
FIGURE 6.17 shows the absorption spectrum of the pigment chlorophyll a, which was isolated from the leaves of Elodea (also known as Anacharis), a common aquarium plant. Also shown is the action spectrum for photosynthetic activity by the same plant. A comparison of the two spectra shows that the wavelengths at which photosynthesis is highest are the same wavelengths at which chlorophyll a absorbs light.
In plants, two chlorophylls absorb light energy to drive the light reactions: chlorophyll a and chlorophyll b. These two molecules differ only slightly in their molecular structures. Both have a complex ring structure, similar to that of the heme group of hemoglobin, with a magnesium ion at the center (FIGURE 6.18). A long hydrocarbon “tail” anchors the chlorophyll molecule to integral proteins in the thylakoid membrane of the chloroplast.
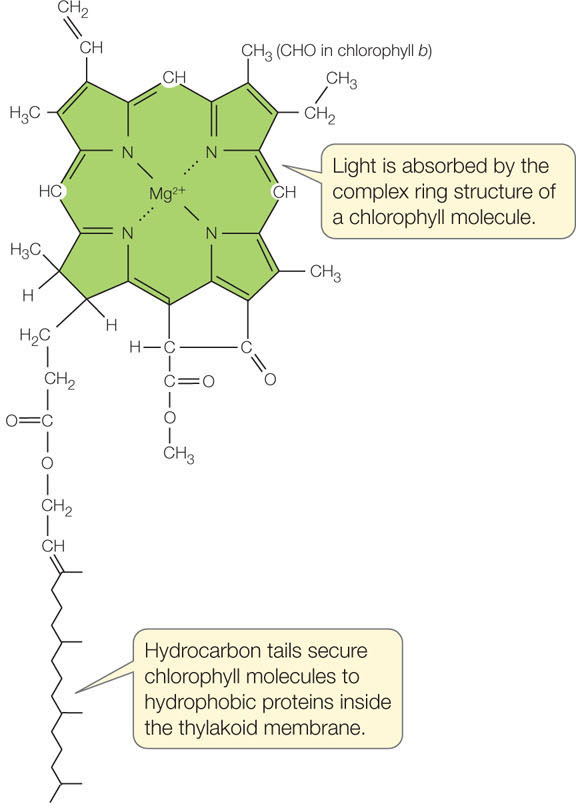
121
As mentioned above, the chlorophylls absorb blue and red light, which are near the two ends of the visible spectrum (see Figure 6.17). In addition, plants possess accessory pigments that absorb photons intermediate in energy between the red and the blue wavelengths, and then transfer a portion of that energy to the chlorophylls. Among these accessory pigments are carotenoids such as β-carotene, which absorb photons in the blue and blue-green wavelengths and appear deep yellow. The phycobilins, which are found in red algae and in cyanobacteria, absorb various yellow-green, yellow, and orange wavelengths.
LINK
Some plant pigments act as sensors that regulate growth and development; see Concept 26.4
Light absorption results in photochemical change
The pigments in photosynthetic organisms are arranged into energy-absorbing antenna systems, also called light-harvesting complexes (FIGURE 6.19A). These form part of a large multiprotein complex called a photosystem, where light energy is converted into chemical energy (FIGURE 6.19B). The photosystem spans the thylakoid membrane and consists of multiple antenna systems with their associated pigment molecules, all surrounding a reaction center.
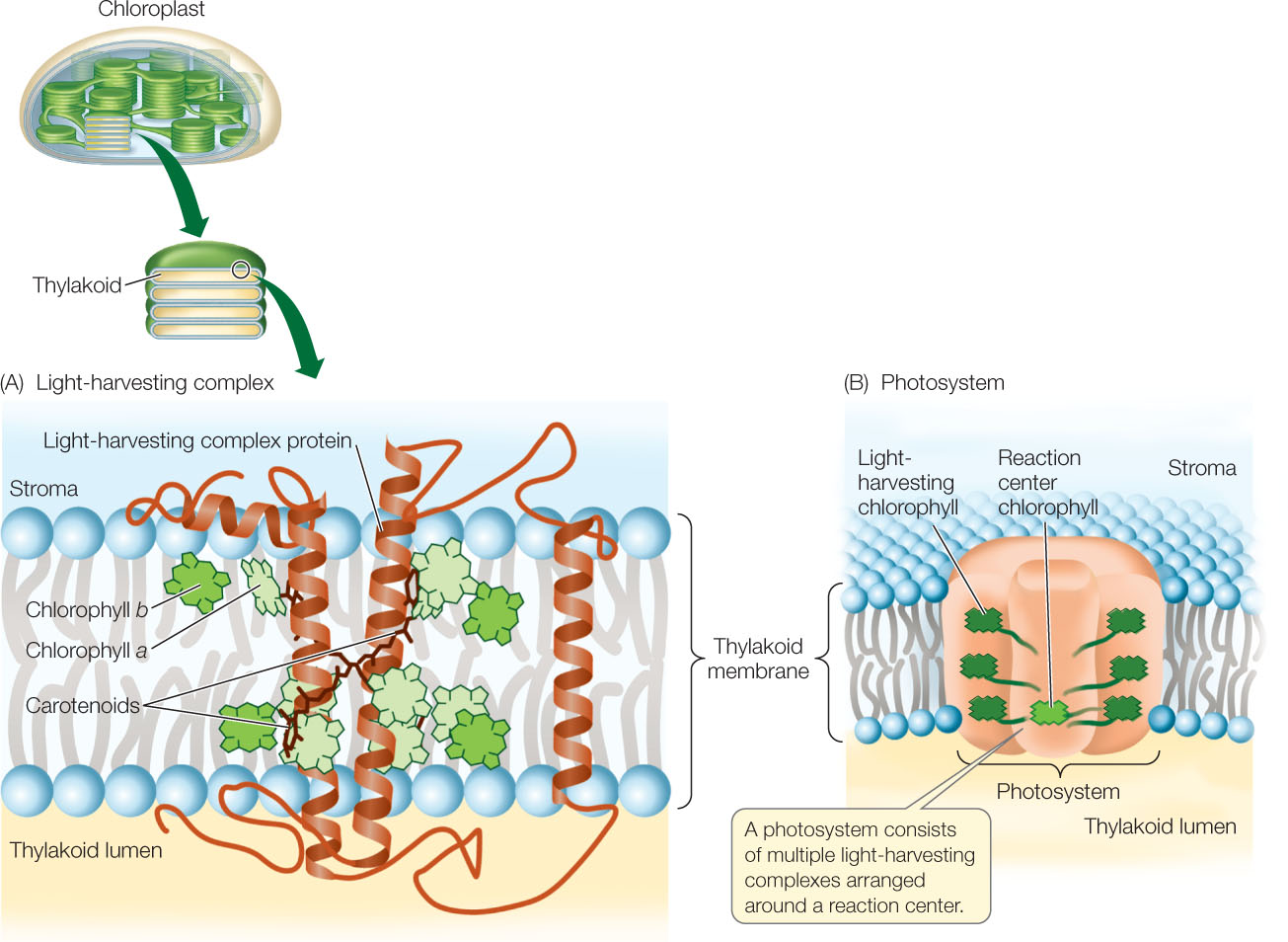
When chlorophyll absorbs light, it enters an excited state. This is an unstable situation, and the chlorophyll rapidly returns to its ground state, releasing most of the absorbed energy. This is an extremely rapid process—measured in picoseconds (trillionths of a second)! For most chlorophyll molecules embedded in the thylakoid membrane, the released energy is absorbed by other, adjacent chlorophyll molecules. The energy eventually arrives at a ground-state chlorophyll molecule at the reaction center (symbolized by Chl), which absorbs the energy and becomes excited (Chl*). But when the reaction center chlorophyll returns to the ground state, something very different occurs. The reaction center converts the absorbed light energy into chemical energy. The chlorophyll molecule in the reaction center absorbs sufficient energy that it actually gives up its excited electron to a chemical acceptor:
122
Chl* + acceptor → Chl+ + acceptor−
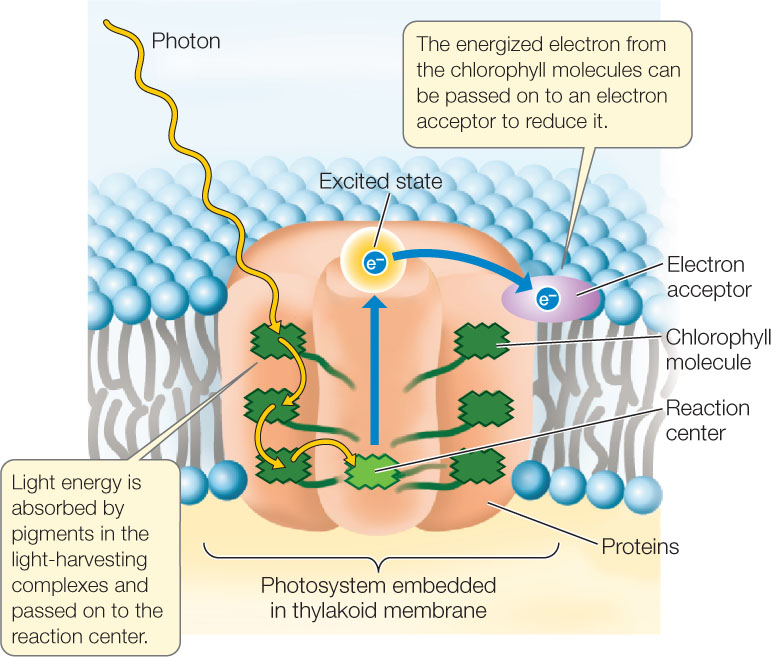
This, then, is the first consequence of light absorption by chlorophyll: the reaction center chlorophyll (Chl*) loses its excited electron in a redox reaction and becomes Chl+. As a result of this transfer of an electron, the chlorophyll gets oxidized, while the acceptor molecule is reduced.
Reduction leads to ATP and NADPH formation
The electron acceptor that is reduced by Chl* is the first of a chain of electron carriers in the thylakoid membrane. Electrons are passed from one carrier to another in an energetically “downhill” series of reductions and oxidations. Thus the thylakoid membrane has an electron transport system similar to the respiratory chain of mitochondria (see Concept 6.2). The final electron acceptor is NADP+, which gets reduced:
NADP+ + H+ + 2 e− → NADPH
As in mitochondria, ATP is produced chemiosmotically during the process of electron transport (a process called photophosphorylation). FIGURE 6.20 shows the series of electron transport reactions that use the energy from light to generate NADPH and ATP. There are two photosystems, each with its own reaction center:
- Photosystem I (containing the “P700” chlorophylls at its reaction center) absorbs light energy at 700 nm and passes an excited electron to NADP+, reducing it to NADPH.
- Photosystem II (with “P680” chlorophylls at its reaction center) absorbs light energy at 680 nm, oxidizes water molecules, and initiates the electron transport chain that produces ATP.
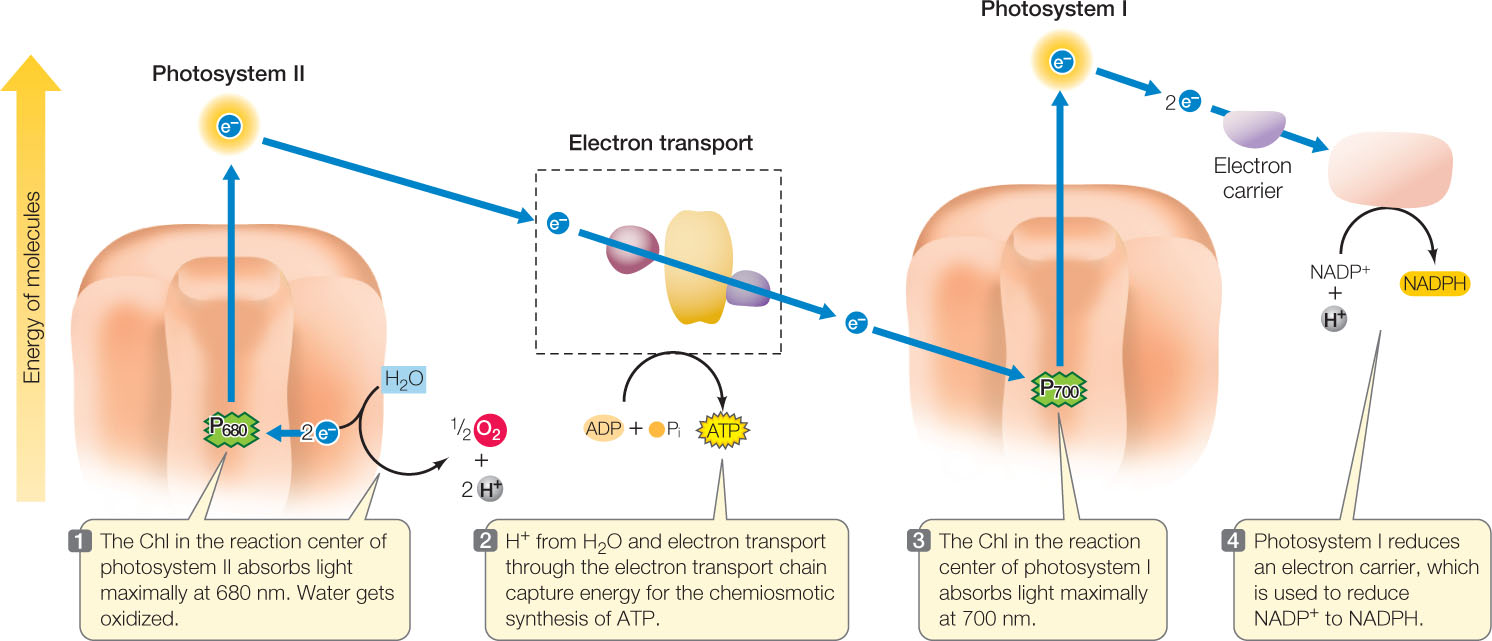
Let’s look in more detail at these photosystems, beginning with photosystem II.
123
Photosystem II
After an excited chlorophyll in the reaction center (Chl*) gives up its energetic electron to reduce a chemical acceptor molecule, the chlorophyll lacks an electron and is very unstable. It has a strong tendency to “grab” an electron from another molecule to replace the one it lost—in chemical terms, it is a strong oxidizing agent. The replenishing electrons come from water, splitting the H—O—H bonds:
H2O → ½ O2 + 2 H+ + 2 e−
2 e− + 2 Chl+ → 2 Chl
Overall: 2 Chl* + H2O → 2 Chl + 2 H+ + ½ O2
The source of the O2 produced by photosynthesis is H2O.
Back to the electron acceptor in the electron transport system: the energetic electrons are passed through a series of thylakoid membrane–bound carriers to a final acceptor at a lower energy level. As in the mitochondrion, a proton gradient is generated and is used by ATP synthase to store energy in the bonds of ATP.
Photosystem I
In photosystem I, an excited electron from the Chl* at the reaction center reduces an acceptor. The oxidized chlorophyll (Chl+) now “grabs” an electron, but in this case the electron comes from the last carrier in the electron transport system. This links the two photosystems chemically. They are also linked spatially, with the two photosystems in the thylakoid membrane. The energetic electrons from photosystem I pass through several molecules and end up reducing NADP+ to NADPH.
Next in the process of harvesting light energy to produce carbohydrates is the series of carbon-fixation reactions. These reactions require more ATP than NADPH. If the pathway we just described—the linear, or noncyclic, pathway—were the only set of light reactions operating, there might not be sufficient ATP for carbon fixation. Cyclic electron transport makes up for this imbalance. This pathway uses only photosystem I and produces ATP but not NADPH; it is cyclic because the electrons flow from the reaction center of photosystem I, through the electron transport chain, and then back to photosystem I (FIGURE 6.21).
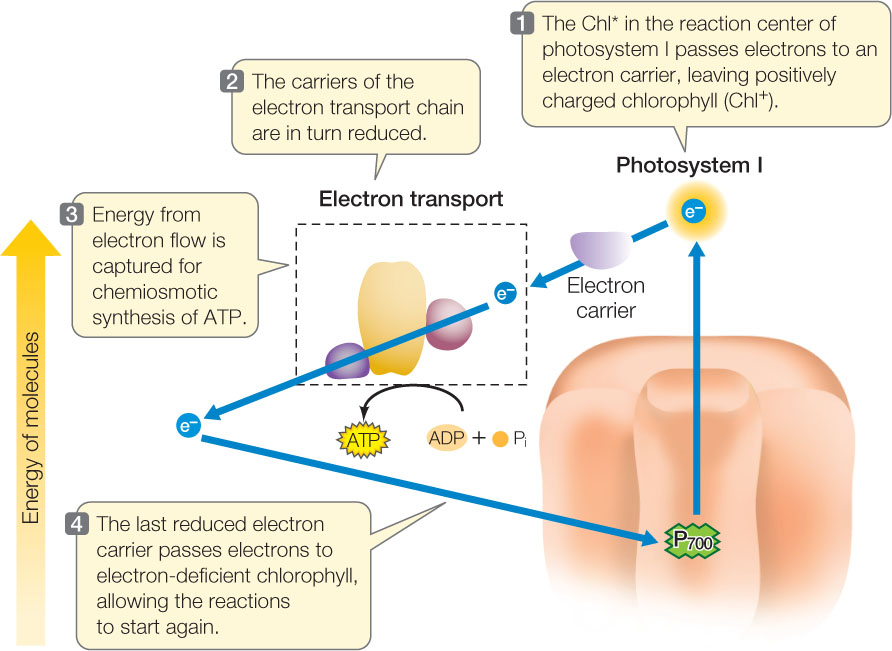
During photosynthesis, light energy is converted to chemical energy
The key role of water in supplying electrons for reduction of light-activated chlorophyll in the light reactions and in the release of O2 to the atmosphere in the process of energy conversion has been investigated using isotopes of oxygen. The 18O isotope is heavier than normal oxygen (16O), and a mass spectrometer can be used to detect the difference. Green plant cells were exposed to light, water, and CO2. (The CO2 was supplied as the bicarbonate ion HCO3−, which forms CO2 when dissolved in water.) In the first experiment, some of the oxygen atoms in the water molecules were 18O (H218O), while CO2 had the normal form of oxygen (C16O2). In the second experiment, the situation was reversed, with H216O and C18O2 being supplied to the cells. After 2 hours of photosynthesis, the ratio of 18O to 16O was measured in the O2 produced by the cells.a
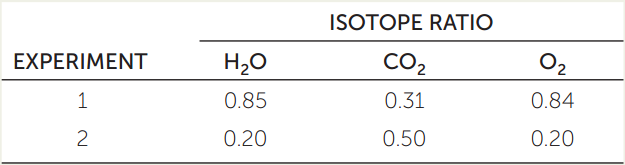
- In experiment 1, was the isotopic ratio of O2 more similar to that of H2O or CO2?
- What about experiment 2? What can you conclude?
a S. Ruben et al. 1941. Journal of the American Chemical Society 63: 877–879.
124
CHECKpointCONCEPT6.5
- What are the reactants and products of the light reactions of photosynthesis?
- Chlorophyll absorbs light of blue and red wavelengths, but leaves also absorb some light at other wavelengths. Explain why.
- Trace the flow of electrons in noncyclic electron transport in the chloroplast and compare it with that of cyclic electron transport.
- Write equations for the production of the following in photosynthesis, and indicate whether they are oxidations, reductions, or neither: Chl*; O2; ATP; NADPH.
We have seen how photosystems I and II absorb light energy, which ultimately ends up as chemical energy in ATP and NADPH. Let’s look now at how these two energy-rich molecules are used in the carbon-fixation reactions to reduce CO2 and thereby form carbohydrates.