CONCEPT6.2 Carbohydrate Catabolism in the Presence of Oxygen Releases a Large Amount of Energy
Cellular respiration is the set of metabolic reactions used by cells to harvest energy from food. Energy is released when reduced organic molecules, with many C—C and C—H bonds, are oxidized to CO2. We will consider in detail only the oxidation (catabolism) of carbohydrates, but bear in mind that cells also obtain energy from the catabolism of other molecules, such as lipids.
The chemical energy released from the complete oxidation of glucose to CO2 is considerable:
Glucose + 6 O2 → 6 CO2 + 6 H2O + energy (686 kcal per mole of glucose)
In a chemistry lab, where sugar is “burned” in the presence of O2, this energy is all lost as heat. In the cell, some of the released energy (234 kcal/mol; 34% of the total) is trapped as ATP. The efficiency of this energy-trapping process is impressive, even when compared with motors that humans have devised. The cell can achieve this through the general principles that govern metabolism listed in Concept 6.1. Most notably, the oxidation occurs in a series of small steps (FIGURE 6.5).
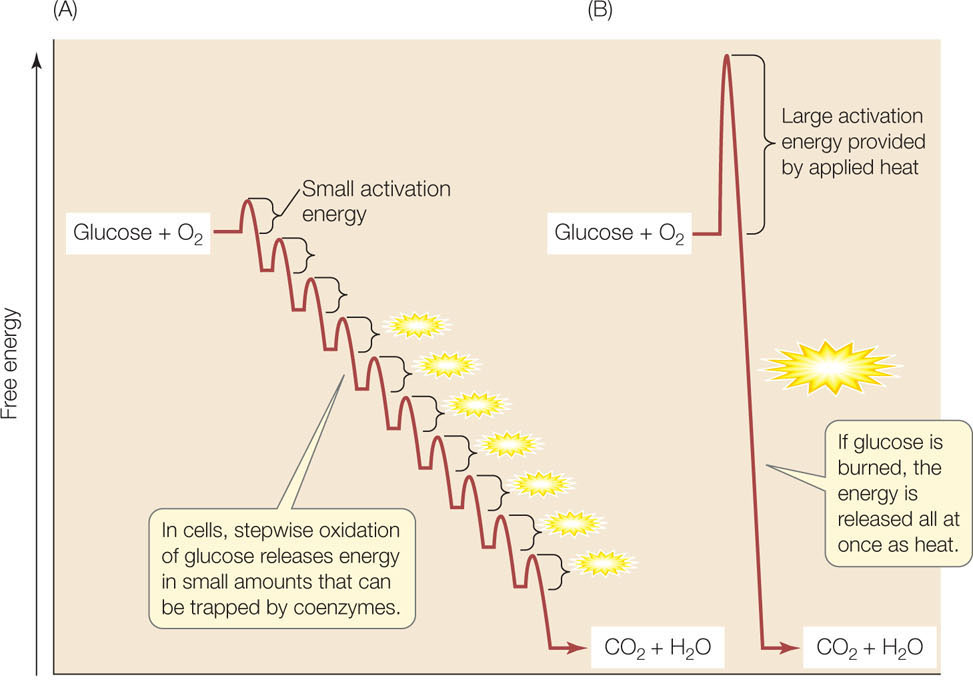
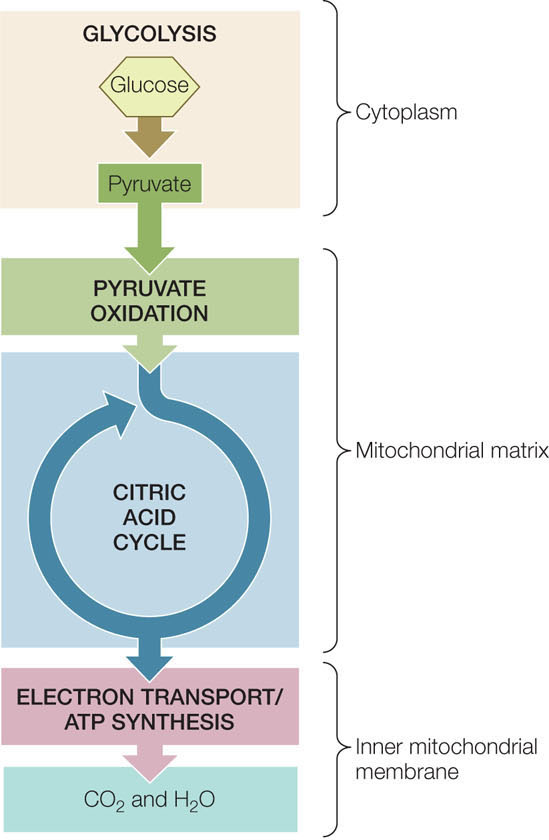
In the catabolism of glucose under aerobic conditions (in the presence of O2), the small steps can be grouped into three linked biochemical pathways (FIGURE 6.6):
- In glycolysis, the six-carbon monosaccharide glucose is converted into two three-carbon molecules of pyruvate.
- In pyruvate oxidation, two three-carbon molecules of pyruvate are oxidized to two two-carbon molecules of acetyl CoA and two molecules of CO2.
- In the citric acid cycle, two two-carbon molecules of acetyl CoA are oxidized to four molecules of CO2.
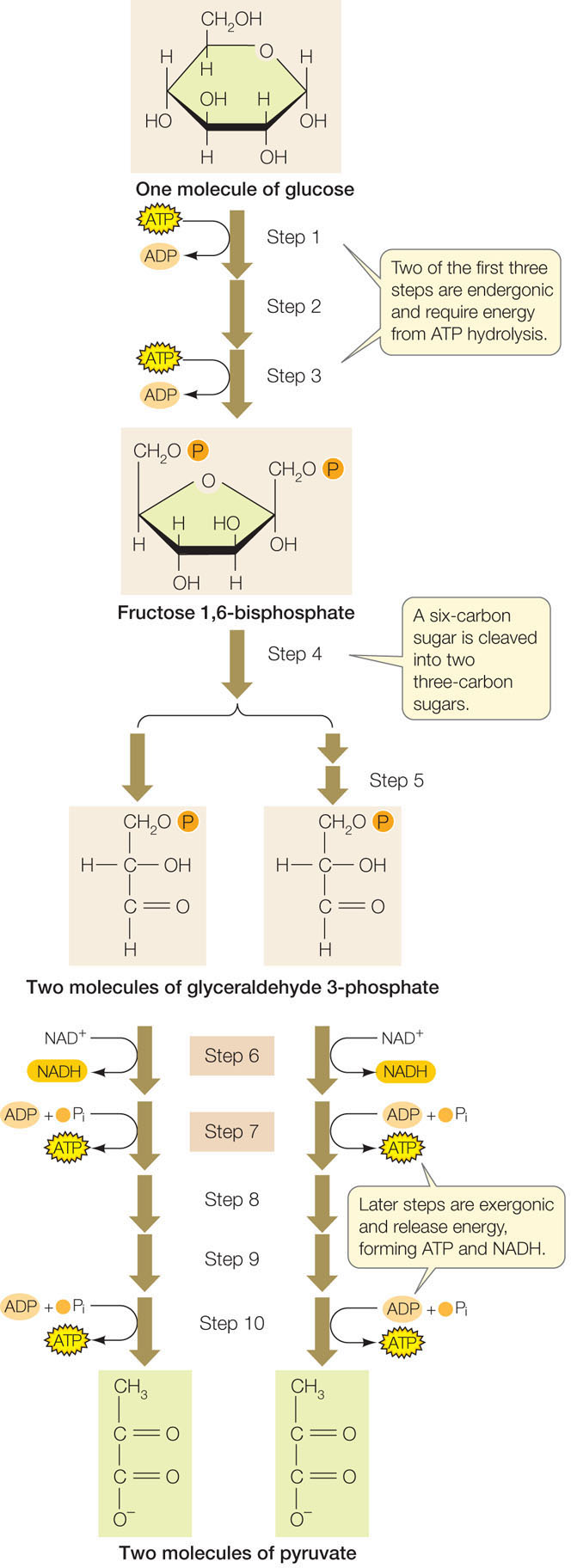
In glycolysis, glucose is partially oxidized and some energy is released
Glycolysis takes place in the cytosol and involves ten enzyme-catalyzed reactions. During glycolysis, some of the C—H bonds in the glucose molecule are oxidized, releasing some stored energy. The final products are two molecules of pyruvate (the anion of pyruvic acid), two molecules of ATP, and two molecules of NADH. Glycolysis can be divided into two stages: the initial energy-investing reactions that consume chemical energy stored in ATP, and the energy-harvesting reactions that produce ATP and NADH (FIGURE 6.7).
110
To help you understand the process without getting into extensive detail, we will focus on two consecutive reactions in this pathway (steps 6 and 7 in Figure 6.7).
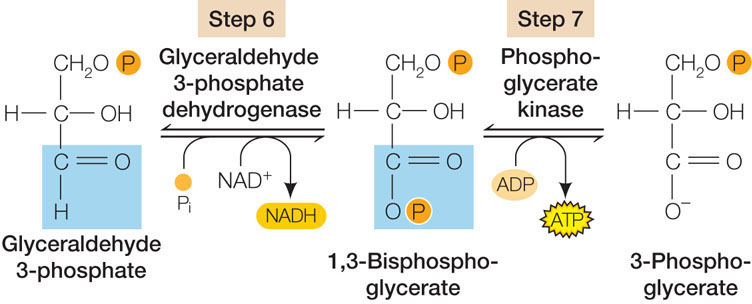
These are examples of two types of reactions that occur repeatedly in glycolysis and in many other metabolic pathways:
- Oxidation–reduction: In the exergonic reaction of step 6, more than 50 kcal/mol of energy are released in the oxidation of glyceraldehyde 3-phosphate. (Look at the first carbon atom, highlighted in blue, where an H is replaced by an O.) The energy is trapped via the reduction of NAD+ to NADH.
- Substrate-level phosphorylation: The second reaction in this series is also exergonic, but in this case less energy is released. It is enough to transfer a phosphate from the substrate (1,3-bisphosphoglycerate) to ADP, forming ATP.
The end product of glycolysis, pyruvate, is somewhat more oxidized than glucose. In the presence of O2, further oxidation can occur. In prokaryotes these subsequent reactions take place in the cytosol, but in eukaryotes they take place in the mitochondrial matrix.
Pyruvate oxidation links glycolysis and the citric acid cycle
The next step in the aerobic catabolism of glucose involves the oxidation of pyruvate to a two-carbon acetate molecule and CO2. The acetate is then bound to coenzyme A (CoA), which is used in various biochemical reactions as a carrier of acetyl groups:
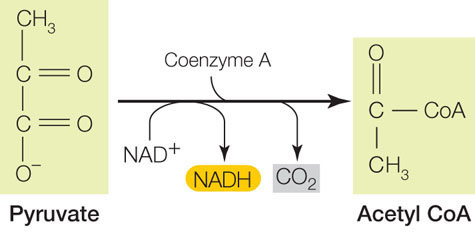
This is the link between glycolysis and further oxidative reactions (see Figure 6.6).
The formation of acetyl CoA is a multistep reaction catalyzed by the pyruvate dehydrogenase complex, which contains 60 individual proteins and 5 different coenzymes. The overall reaction is exergonic, and one molecule of NAD+ is reduced. The main role of acetyl CoA is to donate its acetyl group to the four-carbon compound oxaloacetate, forming the six-carbon molecule citrate (the anion of citric acid). This initiates the citric acid cycle, one of life’s most important energy-harvesting pathways.
111
The citric acid cycle completes the oxidation of glucose to CO₂
Acetyl CoA is the starting point for the citric acid cycle. This pathway of eight reactions completely oxidizes the two-carbon acetyl group to two molecules of CO2. The free energy released from these reactions is captured by ADP and the electron carriers NAD+ and FAD (FIGURE 6.8). This is a cycle because the starting material, oxaloacetate, is regenerated in the last step and is ready to accept another acetate group from acetyl CoA. The citric acid cycle operates twice for each glucose molecule that enters glycolysis (once for each pyruvate that enters the mitochondrion).
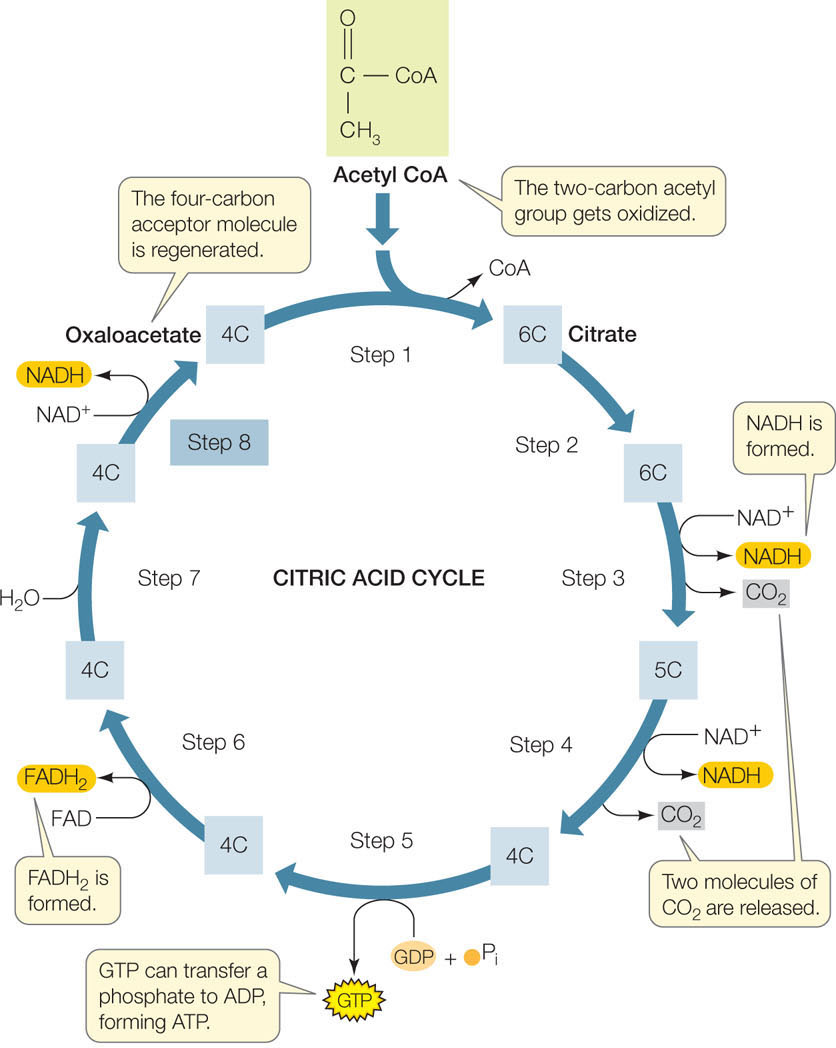
Let’s focus on the final reaction of the cycle (step 8 in Figure 6.8) as an example of the kind of reaction that occurs:
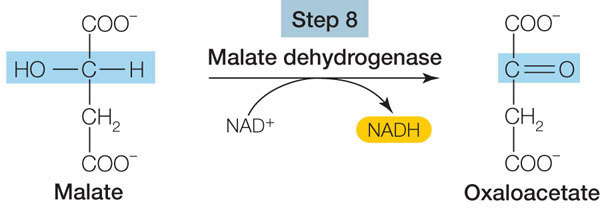
This oxidation reaction (see the blue-highlighted carbon atom) is exergonic, and the released energy is trapped by NAD+, forming NADH. With four such reactions (FADH2 is a reduced coenzyme similar to NADH), the citric acid cycle harvests a great deal of chemical energy from the oxidation of acetyl CoA.
Energy is transferred from NADH to ATP by oxidative phosphorylation
As we mentioned in Concept 6.1, energy-consuming processes in the cell use ATP as their source of energy. In order to fully use the energy harvested in catabolism, cells need to transfer energy from NADH (and FADH2) to the phosphoanhydride bond of ATP. In eukaryotic mitochondria, this transfer is accomplished by oxidative phosphorylation: NADH oxidation is used to actively transport protons (H+ ions) across the inner mitochondrial membrane, resulting in a proton gradient across the membrane. The diffusion of protons back across the membrane is then used to drive the synthesis of ATP. (In prokaryotes, oxidative phosphorylation takes place at the cell membrane.)
First let’s examine how the oxidation of NADH and FADH2 leads to the production of the proton gradient. For example, when NADH is reoxidized to NAD+, O2 is reduced to H2O:
NADH + H+ + ½ O2 → NADM+ + H2O
This does not happen in a single step. Rather, there is a series of redox electron carrier proteins called the respiratory chain embedded in the inner membrane of the mitochondrion (FIGURE 6.9). The electrons from the oxidation of NADH and FADH2 pass from one carrier to the next in the chain in a process called electron transport. The oxidation reactions are exergonic, and they release energy that is used to actively transport H+ ions across the membrane.
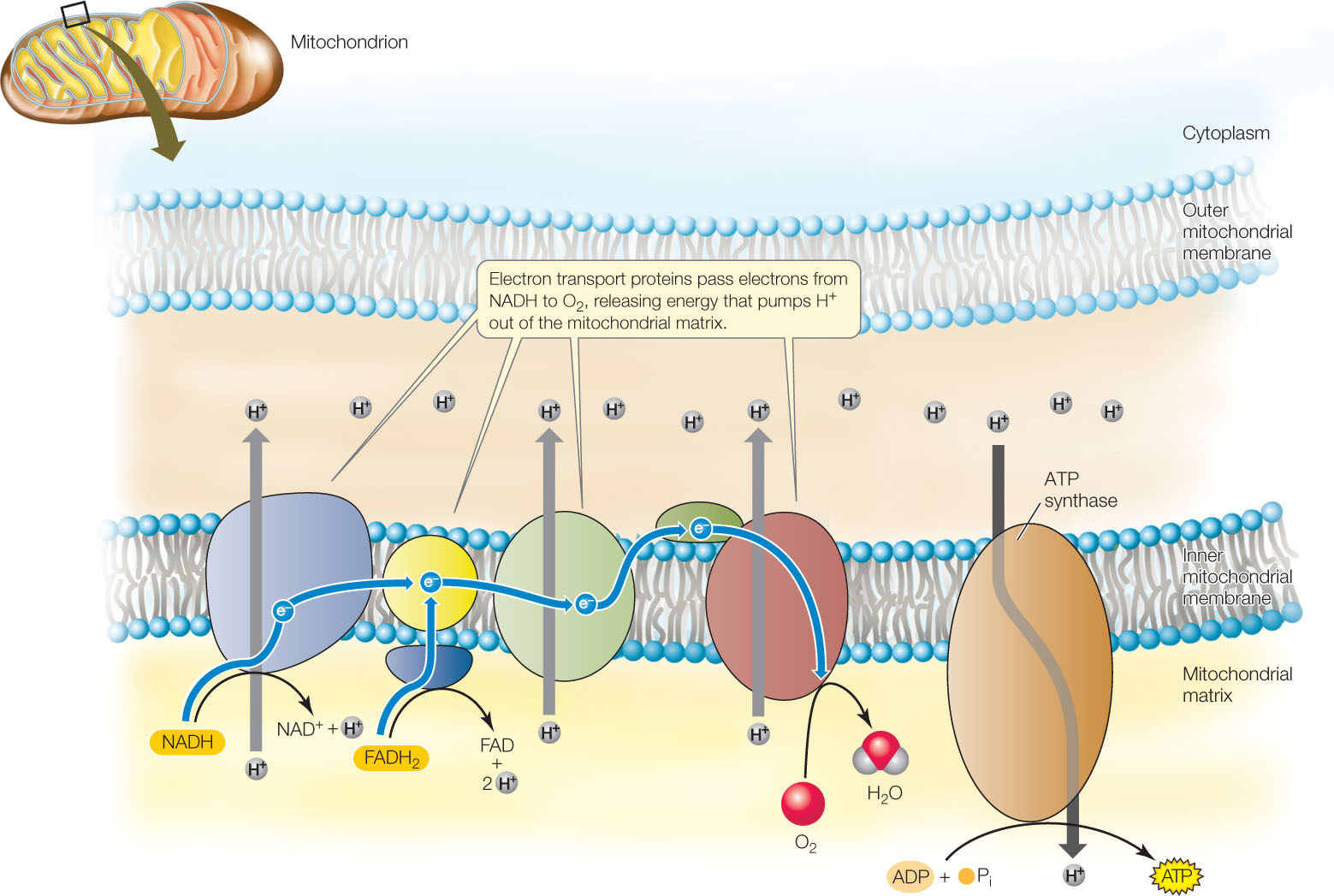
An important aspect of this process is that an oxidation reaction is always coupled with a reduction. When NADH is oxidized to NAD+, the corresponding reduction reaction is the formation of water from O2:
2 H+ + 2 e− + ½ O2 → H2O
So the key role of O2 in cells—the reason we breathe and have a blood system to deliver O2 to tissues—is to act as an electron acceptor and become reduced.
Chemiosmosis uses the proton gradient to generate ATP
In addition to the electron transport carriers, the inner mitochondrial membrane contains an enzyme called ATP synthase (FIGURE 6.10A). This enzyme uses the H+ gradient to drive the synthesis of ATP via a mechanism called chemiosmosis—the movement of ions across a semipermeable barrier from a region of higher concentration to a region of lower concentration. Chemiosmosis relies on concepts covered in earlier chapters:
112
- If the concentration of a substance is greater on one side of a membrane than the other, the substance will tend to diffuse across the membrane to its region of lower concentration (see Concept 5.2).
- If a membrane blocks this diffusion, the substance at the higher concentration has potential energy, which can be converted to other forms of energy (see Concept 2.5).
- Because the interior of a membrane is nonpolar, protons (H+) cannot readily diffuse across the membrane, but they can cross the membrane through the ATP synthase enzyme. ATP synthase converts the potential energy of the proton gradient (called the proton motive force) into the chemical energy in ATP.
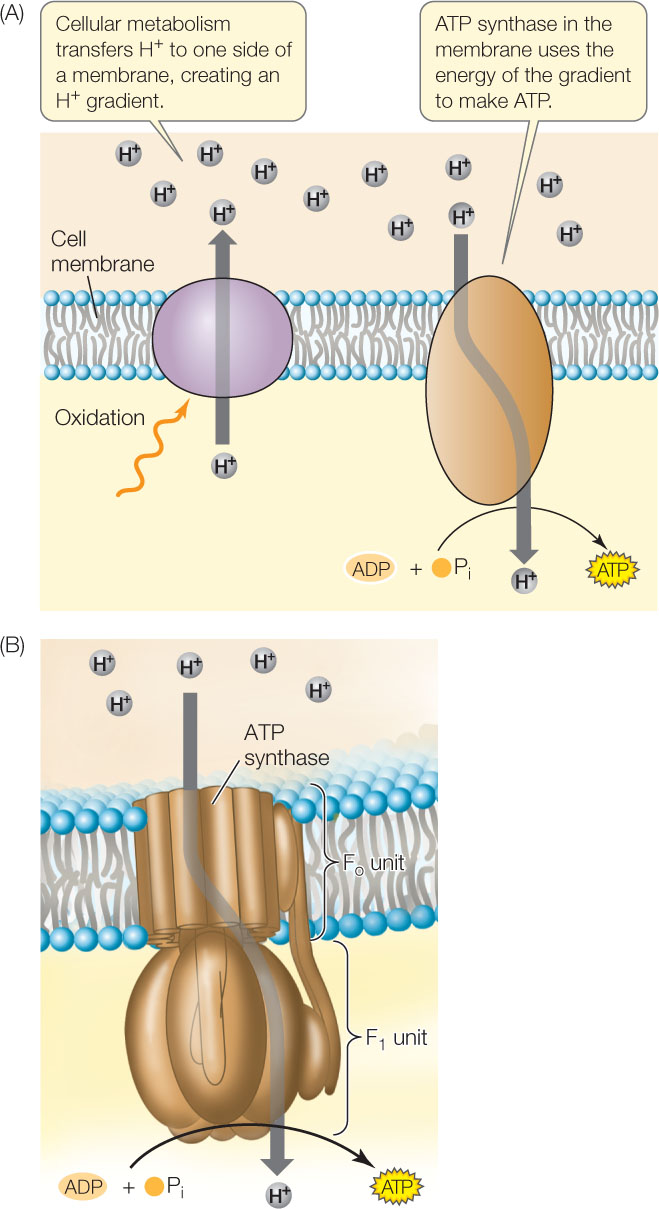
ATP synthase is a molecular motor composed of two parts: the Fo unit, which is a transmembrane domain that functions as the H+ channel; and the F1 unit, which contains the active sites for ATP synthesis (FIGURE 6.10B). The F1 unit consists of six subunits (three each of two polypeptide chains), arranged like the segments of an orange around a central polypeptide. The potential energy set up by the proton gradient drives the passage of protons through the ring of polypeptides that make up the Fo component. This ring rotates as the protons pass through the membrane, causing part of the F1 unit to rotate as well. ADP and Pi bind to active sites that become exposed on the F1 unit as it rotates, and ATP is made.
The structure and function of ATP synthase enzymes are shared by living organisms as diverse as bacteria and humans. These enzymes make ATP at rates of up to 100 molecules per second. In all organisms, these molecular motors rely on protein gradients across membranes:
- In prokaryotes, the gradient is set up across the cell membrane, using energy from various sources.
- In eukaryotes, chemiosmosis occurs in the mitochondria and the chloroplasts.
- As we have just seen, the H+ gradient in mitochondria is set up across the inner mitochondrial membrane, using energy released by the oxidation of NADH and FADH2.
- In chloroplasts, the H+ gradient is set up across the thylakoid membrane using energy from light (see Concept 6.5). In this case, the reduced molecule is NADP+, a relative of NAD+.
Despite these differences in detail, the mechanism of chemiosmosis is similar in almost all forms of life.
Chemiosmosis can be demonstrated experimentally
If chloroplasts or mitochondria are isolated from cells and put in a test tube, a proton gradient can be introduced artificially. This artificial gradient drives ATP synthesis (FIGURE 6.11), but only if ATP synthase, ADP, inorganic phosphate, and the membrane are present.
Investigation
HYPOTHESIS
A H+ gradient across a membrane that contains ATP synthase is sufficient to drive ATP synthesis.
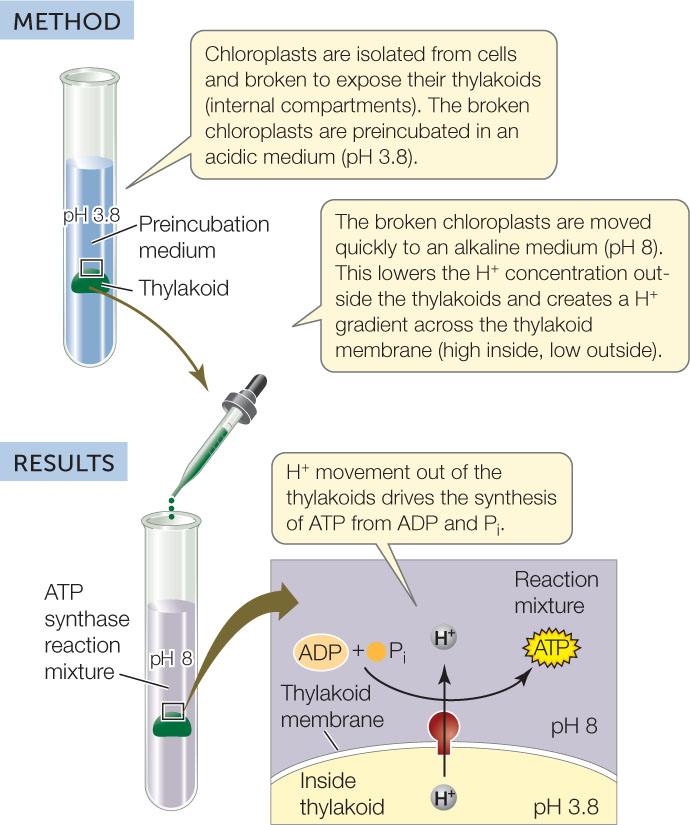
CONCLUSION
A H+ gradient across an ATP synthase–containing membrane is sufficient for ATP synthesis by organelles.
ANALYZE THE DATA
The formation of ATP from ADP and Pi was measured using Luciferase, which catalyzes the formation of a luminescent (light-emitting) molecule if ATP is present. The experiment was performed under different conditions, with the following results:
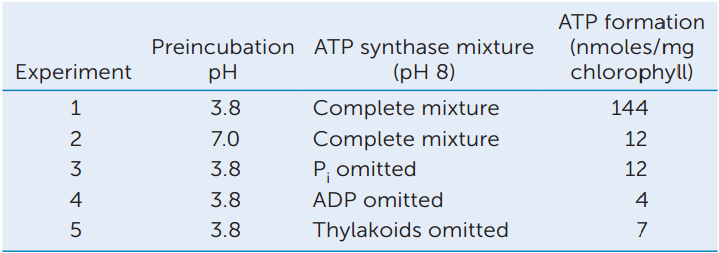
- Which experiments show that a proton gradient is necessary to stimulate ATP formation?
- Why was there less ATP production in the absence of Pi?
a A. T. Jagendorf and E. Uribe. 1966. Proceedings of the National Academy of Sciences USA 55: 170–177.
What happens if the H+ gradient is destroyed by the presence of a membrane channel that is always open to protons? ATP cannot be made, but the oxidation of NADH still occurs and O2 is reduced, releasing considerable energy. The released energy forms heat instead of being used to make ATP. In newborn human infants, a membrane protein appropriately called uncoupling protein 1 disrupts the H+ gradient in fat cell mitochondria, and this results in the release of heat. Because infants lack body hair, this process helps keep them warm.
A popular weight loss drug in the 1930s was the synthetic uncoupler molecule dinitrophenol. There were claims of dramatic weight loss when the drug was administered to obese patients. Unfortunately, the heat that was released caused fatally high fevers, and the effective dose and fatal dose were quite close. The use of this drug was discontinued in 1938, but the general strategy of using an uncoupler for weight loss remains a subject of research.
Oxidative phosphorylation and chemiosmosis yield a lot of ATP
For each NADH (or FADH2) that begins the respiratory chain, two to three (let’s say 2.5) ATP molecules are formed under the conditions in the cell. Thus the four molecules of reduced coenzyme produced by each turn of the citric acid cycle yield about ten (4 × 2.5) molecules of ATP. Two molecules of acetyl CoA are produced from each glucose, so the total is about 20 ATPs per molecule of glucose. Add to this the NADH produced by glycolysis and pyruvate oxidation, and the ATP formed by substrate-level phosphorylation during glycolysis and the citric acid cycle, and the total is about 32 molecules of ATP produced per fully oxidized glucose.
The vital role of O2 is now clear: most of the ATP produced in cellular respiration is formed by oxidative phosphorylation—the process of transferring electrons from NADH to O2, resulting in the reoxidation of NADH to NAD+. The accumulation of atmospheric O2 as a result of photosynthesis by ancient microorganisms (see Concept 18.2) set the stage for the evolution of oxidative phosphorylation; organisms that could exploit the O2 would have had a selective advantage.
Nevertheless, many microorganisms still thrive where O2 is scarce. These anaerobic bacteria and archaea use alternative electron acceptors in their natural environments. For instance, the bacterium Geobacter metallireducens typically lives in sediments under streams or ponds, and uses metal ions as terminal electron acceptors. For example:
Fe3+ + e− (from electron transport) → Fe2+
This bacterium can also use radioactive uranium ions as electron acceptors. In the process, the uranium is converted from a soluble to an insoluble form, making Geobacter of potential use in environmental cleanup. The bacterium can convert uranium in contaminated water into a form that accumulates in the sediment instead and can be more readily removed.
114
Carbohydrate catabolism in the presence of oxygen releases a large amount of energy
The following reaction occurs in the citric acid cycle:
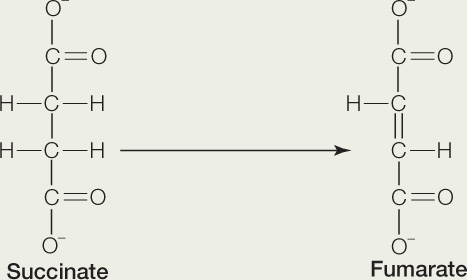
Answer each of the following questions, and explain your answers:
- Is this reaction an oxidation or reduction?
- Is the reaction exergonic or endergonic?
- This reaction requires a coenzyme. What kind of coenzyme?
- What happens to the fumarate after the reaction is completed?
- What happens to the coenzyme after the reaction is completed?
115
CHECKpointCONCEPT6.2
- Draw the molecules of glucose and pyruvate and compare them with respect to their oxidation/reduction states. What does this mean in terms of available chemical bond energy?
- Compare the energy yields (in terms of ATP per glucose) from glycolysis and from the combination of pyruvate oxidation and the citric acid cycle.
- High levels of ammonia (NH3) are toxic to mammals. One reason is that ammonia bonds with α-ketoglutarate (the substrate for step 4 in Figure 6.8), forming the amino acid glutamate. This removes α-ketoglutarate from the citric acid cycle. Explain the consequence of this for the cell.
- Hibernating animals usually have low rates of metabolism. Periodically, they have episodes of arousal, where they rapidly increase body temperature. During arousal, they synthesize a membrane protein not normally present in their cells. What might that protein do?
We have seen that a large amount of energy is released when carbohydrates are catabolized in the presence of O2. If O2 is absent, the yield of ATP is much lower. We will now turn to this situation.