CONCEPT39.5 The Adaptive Cellular Immune Response Involves T Cells and Their Receptors
Two types of effector T cells (T-helper cells and cytotoxic T cells) are involved in the cellular immune response, along with proteins of the major histocompatibility complex (MHC proteins), which underlie the immune system’s tolerance for the body’s own cells.
T cell receptors specifically bind to antigens on cell surfaces
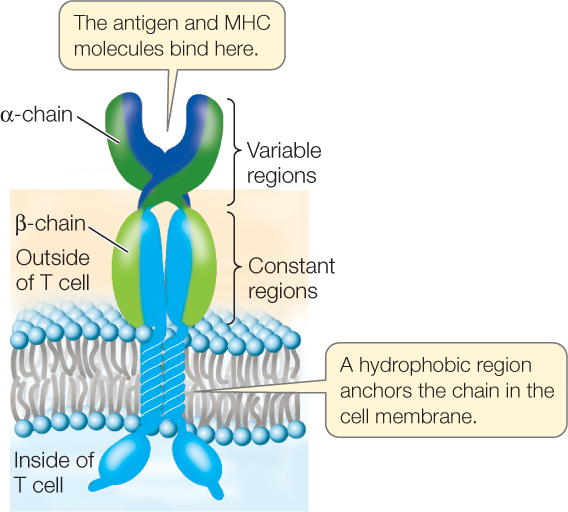
Like B cells, T cells possess specific membrane receptors. The T cell receptor is not an immunoglobulin, however, but a glycoprotein with a molecular weight of about half that of an IgG. It is made up of two polypeptide chains, each encoded by a separate gene (FIGURE 39.11). The two chains have distinct regions with constant and variable amino acid sequences. As in the immunoglobulins, the variable regions provide the site for specific binding to antigens. However, there is an important difference between immunoglobulin binding and T cell receptor binding: whereas an immunoglobulin can bind to an antigen whether it is present on the surface of a cell or not, a T cell receptor binds only to an antigen displayed by an MHC protein on the surface of an antigen-presenting cell.
MHC proteins present antigens to T cells and result in recognition
Recall that so far we have described two types of T cells: TH and TC cells. Both cell types express T cell receptors that bind to antigen on the cell surface. But the response of each cell type to binding is quite different. TH binding results in activation of the adaptive immune response, whereas TC binding results in death of the cell carrying the antigen. MHC proteins form complexes with antigens on cell surfaces and assist with recognition by the T cells, so that the appropriate type of T cell binds.
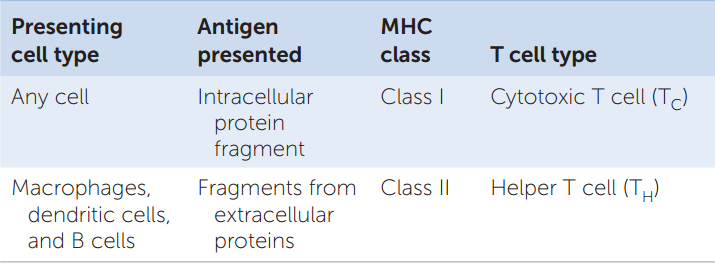
The MHC proteins are cell membrane glycoproteins. Two types of MHC proteins function to present antigens to the two different types of T lymphocytes:
- Class I MHC proteins are present on the surface of every nucleated cell in the mammalian body. They present antigens to TC cells. These antigens can be fragments of virus proteins in virus-infected cells or abnormal proteins made by cancer cells as a result of somatic mutations.
- Class II MHC proteins are on the surfaces of macrophages, B cells, and dendritic cells. They present antigens to TH cells. The three cell types ingest antigens and break them down; one of the fragments then binds to MHC II for presentation (FIGURE 39.12).
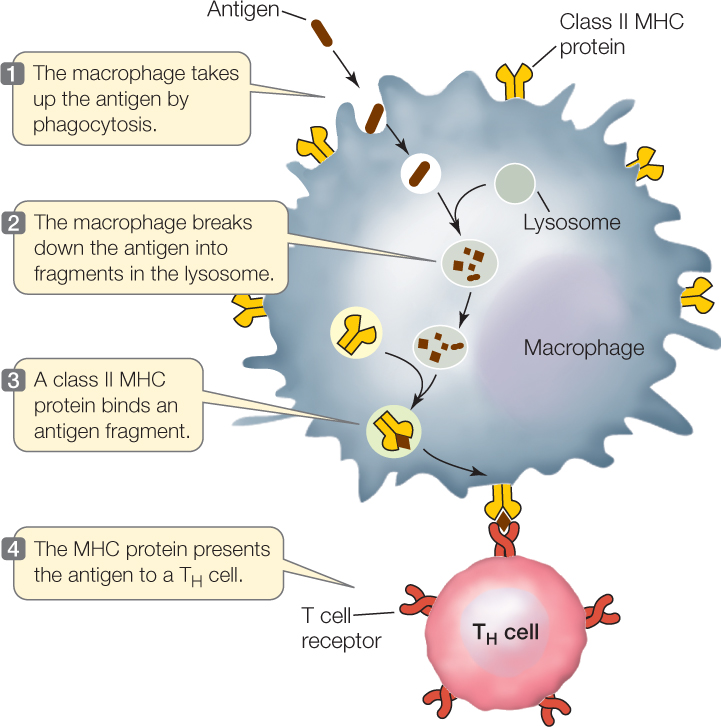
In humans, there are three genetic loci for class I MHC proteins and three for class II MHC proteins. Each of these six loci has as many as 1,000 different alleles. With so many possible allele combinations, it is not surprising that different people are very likely to have different MHC genotypes. This is why it can be difficult to find a good “match” for organ donations. MHC proteins are “self” markers. To accomplish its role in antigen presentation, an MHC protein has an antigen-binding site that can hold a peptide of about 10–20 amino acids. The T cell receptor recognizes not just the antigenic fragment but also the class I or II MHC molecule to which the fragment is bound.
823
Information on MHC proteins, the cellular origins of antigens, and T lymphocytes is summarized in TABLE 39.2.
TH cells contribute to the humoral and cellular immune responses
A TH cell is activated when it has receptors that can bind a specific antigen on the surface of an antigen-presenting cell. This results in the rapid clonal propagation of the TH cell. The TH cells then release cytokines that stimulate other immune cells, including TC cells, to propagate.
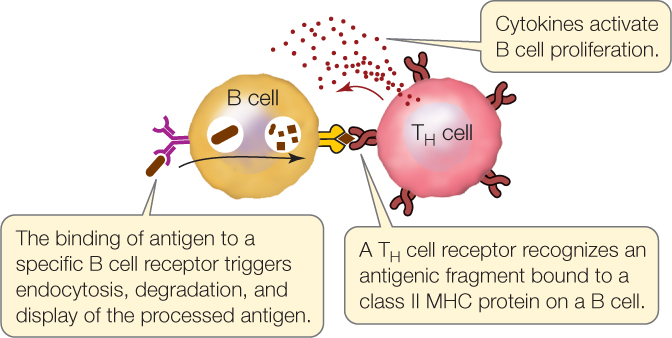
A TH cell can also directly interact with a B cell that recognizes the same antigen. Like dendritic cells and macrophages, B cells are antigen-presenting cells. B cells take up antigens bound to their surface immunoglobulin receptors by endocytosis, break them down, and display antigenic fragments on class II MHC proteins. When a TH cell binds to the displayed antigen–MHC II complex, it stimulates the B cell to propagate and to secrete antibodies.
Activation of the cellular response results in death of the targeted cell
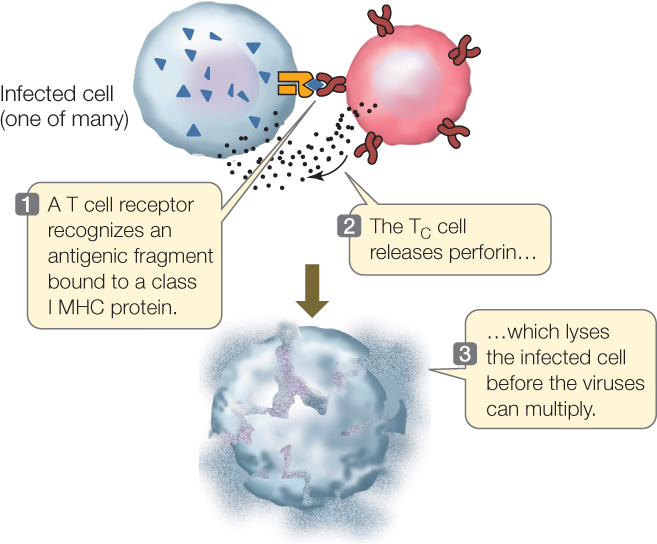
TC cells are activated when they bind to cells carrying an antigen-MHC I protein complex, such as a virus-infected cell. This activation, along with signals from TH cells, results in the production of a clone of TC cells with the same specific receptor. When bound, the TC cells do two things to eliminate the antigen-carrying cell:
- They produce perforin, which lyses the bound target cell.
- They stimulate apoptosis in the target cell.
824
Regulatory T cells suppress the humoral and cellular immune responses
A third class of T cells called regulatory T cells (Tregs) ensures that the immune response does not spiral out of control. Like TH and TC cells, Tregs are made in the thymus gland, express the T cell receptor, and become activated if they bind to antigen–MHC protein complexes. But Tregs are different in one important way: the antigens that Tregs recognize are self antigens. The activation of Tregs causes them to secrete the cytokine interleukin-10, which blocks T cell activation and leads to apoptosis of the TC and TH cells that are bound to the same antigen-presenting cell (FIGURE 39.13).
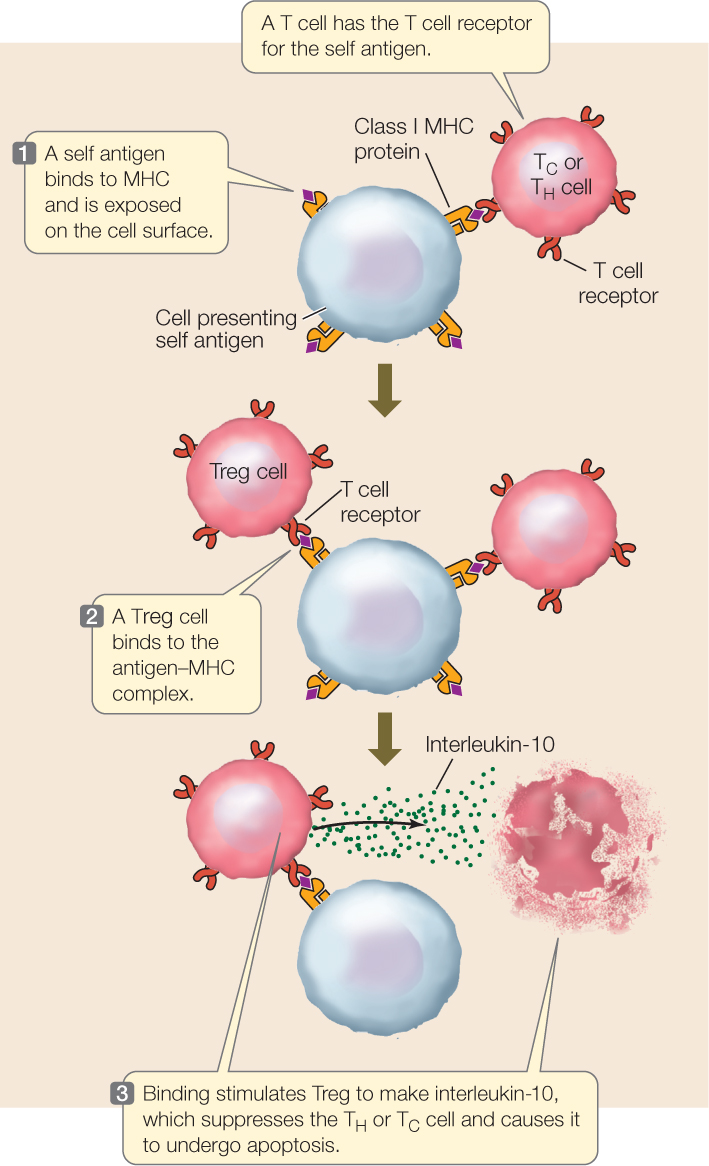
The important role of Tregs is to mediate tolerance to self antigens. Thus they constitute one of the mechanisms for distinguishing self from nonself. There are two lines of experimental evidence for the role of Tregs:
- If Tregs are experimentally destroyed during development in the thymus of a mouse, the mouse grows up with an out-of-control immune system, mounting strong immune responses to self antigens—autoimmunity.
- In humans, a rare X-linked inherited disease occurs when a gene critical to Treg function is mutated. An infant with this disease, called IPEX (immune dysregulation, polyendocrinopathy and enteropathy, X-linked), mounts an immune response that attacks the pancreas, thyroid, and intestine. Most affected individuals die within the first few years of life.
AIDS is an immune deficiency disorder
There are a number of inherited and acquired immune deficiency disorders. In some individuals, T or B cells never form; in others, B cells lose the ability to give rise to plasma cells. In either case, the affected individual is unable to mount an immune response and thus lacks a major line of defense against pathogens. A more common disease that was first detected in the early 1980s is acquired immune deficiency syndrome (AIDS), which results from infection by human immunodeficiency virus (HIV).
The course of HIV infection provides a good review of adaptive immunity (see Figure 39.7). HIV is transmitted from person to person in blood, semen, vaginal fluid, or breast milk; the recipient tissue is either blood (by transfusion or injection) or a mucous membrane lining an organ. HIV initially infects macrophages, TH cells, and antigen-presenting dendritic cells in the blood and tissues. At first there is an immune response to the viral infection, and some TH cells are activated. But because HIV infects the TH cells, they are killed both by HIV itself and by TC cells that lyse infected TH cells. Consequently, TH cell numbers decline after the first month or so of infection. Meanwhile, the extensive production of HIV by infected cells activates the humoral immune system. Antibodies bind to HIV and the complexes are removed by phagocytes. The HIV level in blood goes down. There is still a low level of infection, however, mostly because of the depletion of TH cells (FIGURE 39.14).
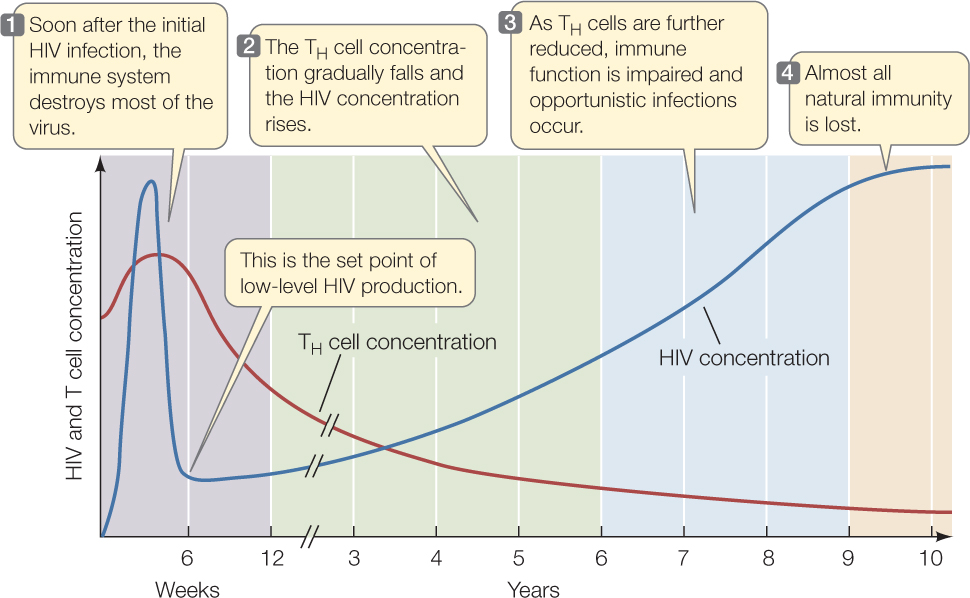
During this dormant period, people carrying HIV generally feel fine, and their TH cell levels are adequate for them to mount immune responses against other infections. Eventually, however, the virus destroys the TH cells, and their numbers fall to the point where the infected person is susceptible to infections that the TH cells would normally eliminate. These infections result in conditions such as Kaposi’s sarcoma, a skin tumor caused by a herpes virus; pneumonia caused by the fungus Pneumocystis jirovecii; and lymphoma tumors caused by the Epstein–Barr virus. These conditions result from opportunistic infections because the pathogens take advantage of the crippled immune system of the host. In addition, HIV infection somehow stimulates Treg cells, and these also downregulate the immune response. The combination of infections and a weakened immune response can lead to death within a year or two of development of full-blown AIDS.
825
The molecular biology of HIV and its life cycle have been intensively studied. Drug treatments are focused on inhibiting processes necessary for viral entry, assembly, and replication, such as the synthesis of cDNA from viral RNA by reverse transcriptase, and the cutting of the large precursor viral protein into mature, active proteins by viral protease. Combinations of such drugs result in long-term survival, a major achievement of molecular biology applied to medicine. Unfortunately, like many medical treatments, HIV drugs are not available to all who need them—particularly in poor regions of the world where AIDS is prevalent. As a result, there are about 1.7 million deaths per year worldwide from AIDS.
LINK
For more on the life cycle of HIV, see Concept 11.2
CHECKpointCONCEPT39.5
- Compare the T cell receptor and B cell receptor in terms of structure, diversity, and function.
- What are the similarities and differences in function between class I and II MHC proteins?
- What are the roles of TH cells in cellular and humoral immunity?
- Since MHC proteins are highly variable and almost always differ among unrelated people, an organ transplant from one person to another will generally provoke a cellular immune response, and the organ will be rejected. Patients receiving organ transplants are treated with cyclosporin, a drug that inhibits T cell development. How do you think cyclosporin prevents rejection? What side effects might you expect in treated people?
How can a person survive an infection and be resistant to further infection?
ANSWER Almost 2,500 years after Thucydides’s observations, we have some answers to this question. During the Athenian plague, innate immunity (Concept 39.2) kept most of the disease agent out of the body. Any agent that evaded the innate defenses was attacked by the adaptive immune system (Concepts 39.3–39.5). The agent was engulfed by macrophages or dendritic cells, which broke up the agent and presented fragments on its cell surface, in complexes with class II MHC proteins. The antigen-presenting cell migrated to a lymph node, where the MHC–antigen complex was recognized by TH cells with the appropriate T cell receptors. Clones of these T cells developed, and some of these cells bound to B cells that displayed the same antigen on their surfaces. The B cells then formed clones of plasma cells, which made antibodies that bound to the agent. The antibody-bound agent was then destroyed by the complement system and phagocytes. Meanwhile, if cells were infected by the agent, a parallel series of events occurred in the cellular immune response, resulting in a clone of TC cells that killed the infected cells (Concept 39.5). These two systems allowed Thucydides to survive the infection that killed so many of his fellow Athenians.
The T and B cells activated by the two adaptive immune responses also formed smaller clones of memory cells. When Thucydides took care of others in Athens who had the disease, the reentry of the pathogen into his body provoked a rapid, massive T and B cell response. This response prevented the pathogen from proliferating and making him sick again.
An important application of immunological memory is the use of vaccines. Exposure to an antigen in a form that does not cause disease can still initiate a primary immune response, generating memory cells without making the person ill. Later, if a pathogen carrying the same antigen attacks, specific memory cells already exist. They recognize the antigen and quickly overwhelm the invaders with a massive production of lymphocytes and antibodies (FIGURE 39.15).
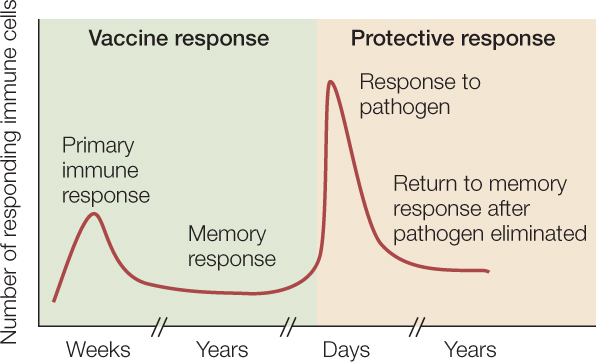
Because the antigens used for immunization or vaccination are derived from pathogenic organisms, they must be altered so they cannot cause disease. This is achieved in a variety of ways, including inactivation of the pathogen by chemicals or heat, mutations that render the pathogen inactive, or recombinant DNA technology to make nonharmful peptides derived from the pathogen. The use of vaccines, especially in children, has been a great success in public health. In industrialized countries, widespread vaccination has completely or almost completely wiped out some deadly diseases, including smallpox, diphtheria, and polio.
826