OVERVIEW OF THE LIPIDS
LIPIDS structurally diverse group of naturally occurring molecules that are generally insoluble in water, but are soluble in organic solvents; examples include fatty acids, triglycerides, sterols, and phospholipids
Scientists have long known that what we commonly call “fats” are a subclass of lipids, a group of compounds made up of carbon, hydrogen, and a small amount of oxygen that generally can’t mix or dissolve in water (they are water insoluble).
Lipids play important roles in the body, such as acting as a major component of cell membranes, giving them flexibility and integrity. They also facilitate the transport of nutrients, including fat-
Fats are a concentrated source of energy, providing 9 kcal per gram, typically accounting for one-
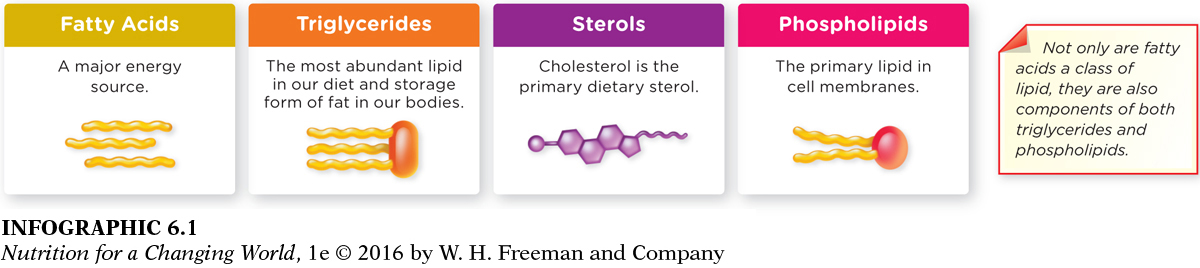
Lipids are diverse in structure and function. We will discuss the four most common lipid classes: fatty acids, triglycerides, sterols (such as cholesterol), and phospholipids (such as lecithin). Although some use the term “lipid” interchangeably with “fat,” the word “fat,” more precisely, refers to triglycerides, which make up 95% of all lipids in our foods and 99% of the stored fat in our bodies. (INFOGRAPHIC 6.1)
Question 6.1
 Which of the lipid classes has a distinctly different structure from the others?
Which of the lipid classes has a distinctly different structure from the others?
Sterols have a different structure from fatty acids, triglycerides, and phospholipids.

It’s no wonder the Inuit often enjoyed blubbery seal meat and other fatty foods—
Dietary fat confers some health benefits, but because many tasty foods are also fatty foods, it can be easy to eat too much fat. Any excess fat in the diet is efficiently deposited as fat in the body (adipose tissue), making high-
When Dyerberg and Bang made their historic trip to Greenland, they were eager to learn about the fish-
Fatty Acids
FATTY ACID a chain of carbon atoms with hydrogen atoms attached, includes a carboxyl group on one end and a methyl group on the other
CARBOXYL GROUP the acid group attached to one end of the fatty acid chain
METHYL GROUP a group of three hydrogen atoms bonded to a carbon atom found at one end (the “omega” end) of the fatty acid chain
Fatty acids are a type of lipid, but they are also the primary components of both triglycerides and phospholipids. Fatty acids consist of a chain of carbon atoms with hydrogen atoms attached to each carbon atom (a hydrocarbon chain): a carboxyl group attached to one end of the fatty acid chain, and a methyl group at the other end (three hydrogens bonded to a carbon atom—
Fatty acids differ in length, and so they are categorized by the length of their hydrocarbon chains, as well as in their degree of saturation (how many hydrogen atoms fill the available bonds with carbon). Short-
SATURATED FATTY ACID a fatty acid that contains no double bonds between carbons in the carbon chain and carries the maximum number of hydrogen atoms
UNSATURATED FATTY ACIDS a fatty acid that has at least one double bond between carbons in the carbon chain and has fewer than the maximum possible number of hydrogen atoms
MONOUNSATURATED FATTY ACID a fatty acid with only one double bond between carbons in the carbon chain
POLYUNSATURATED FATTY ACID a fatty acid with two or more double bonds between carbons in the carbon chain
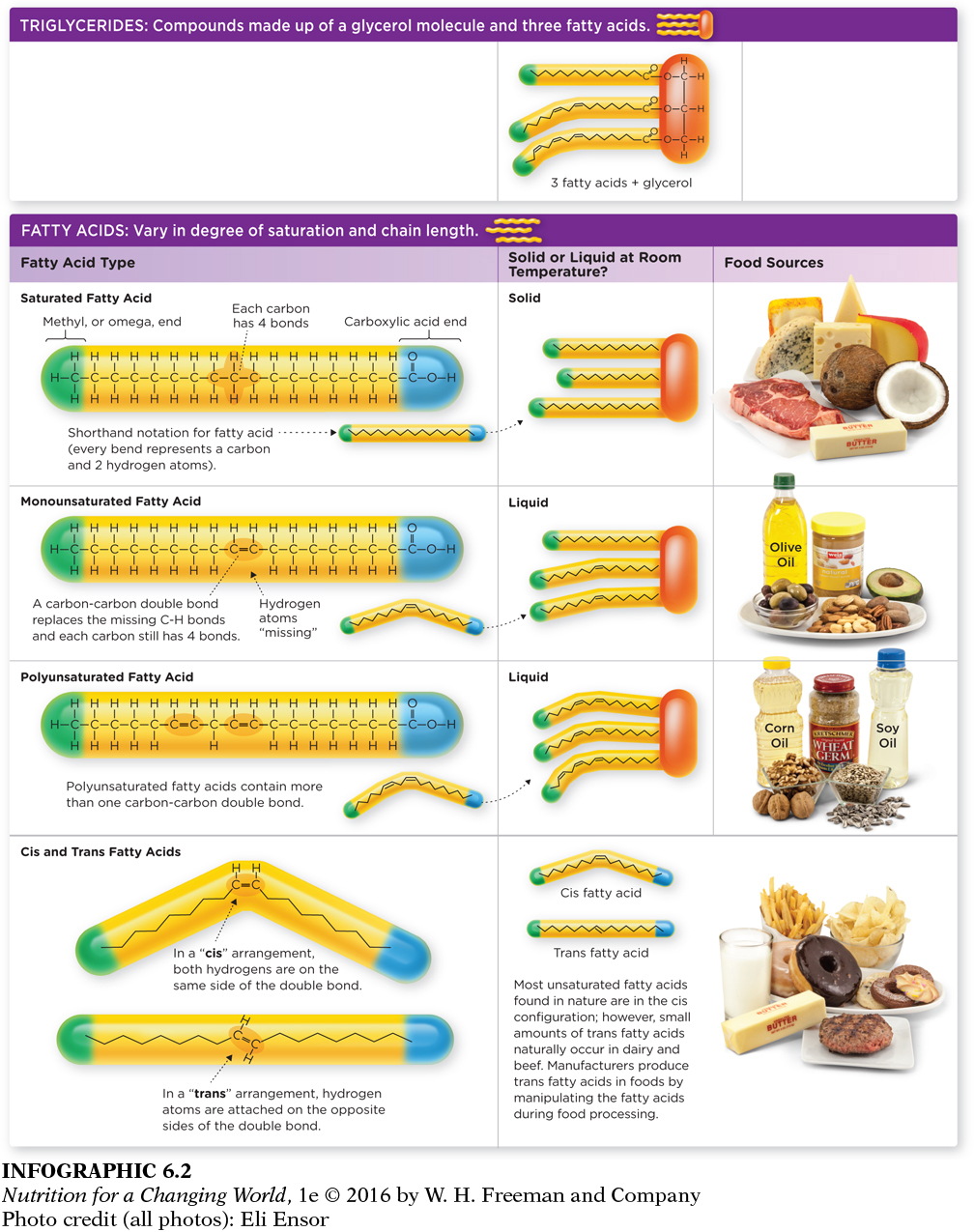
Some fatty acids are saturated, with hydrogen atoms filling every possible bond with carbon atoms. Saturated fatty acids are relatively solid at room temperature, and most commonly found in animal products (such as meats and dairy), as well as some vegetable products (such as coconut oil, palm kernel oil, palm oil, and cocoa butter). The rest are unsaturated, with less hydrogen and one or more double bond (or point of unsaturation) between carbon atoms, and are generally liquid at room temperature. Unsaturated fats are found most abundantly in plant foods, such as seeds, nuts, grains, and most vegetable oils. Fatty acids with one point of unsaturation are called monounsaturated fatty acids (abundant in olive and canola oils and nuts); those with more than one point of unsaturation are called polyunsaturated fatty acids (abundant in corn, safflower, sunflower, sesame, and soybean oils). The arrangement of the hydrogen atoms on either side of the double bonds can be in either a “cis” or a “trans” orientation, which has important health implications, as will be discussed later in the chapter. (INFOGRAPHIC 6.2)
Question 6.2
 How are trans fatty acids both similar to and different from saturated and unsaturated fatty acids?
How are trans fatty acids both similar to and different from saturated and unsaturated fatty acids?
A trans fatty acid is similar to a saturated and unsaturated fatty acid in that it is made up of carbon and hydrogen, and it contains a methyl and carboxylic acid end. However, a saturated fatty acid has the maximum possible number of hydrogen atoms attached to every carbon atom, and mono-
Triglycerides
TRIGLYCERIDES storage form of fat, made up of three fatty acid chains attached to the three carbons on a glycerol molecule
GLYCEROL a three-
Triglycerides are lipids made up of three fatty acid chains bound to one glycerol, a small three-
Triglycerides in foods supply energy and may also carry certain fat-
Question 6.3
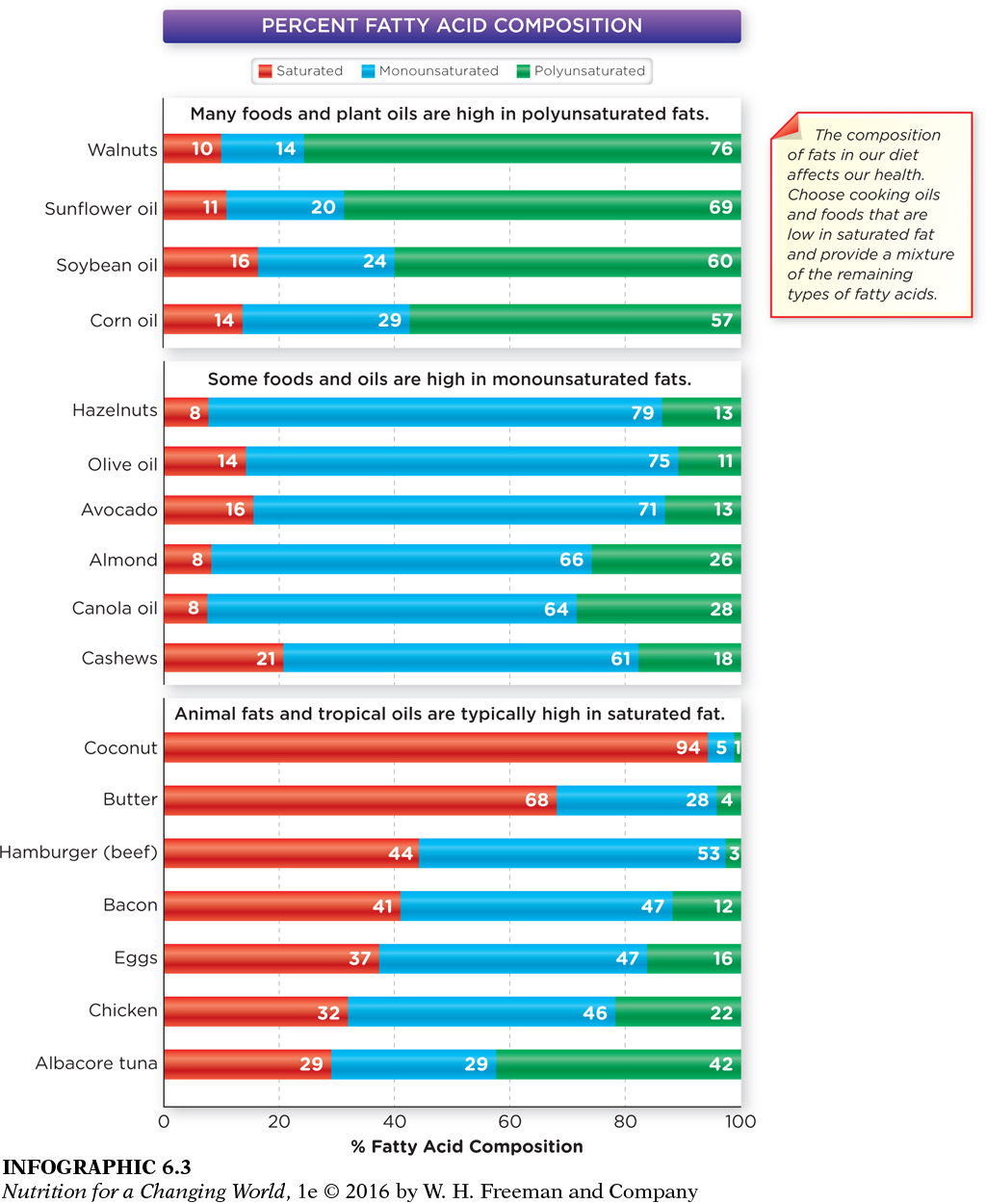
 Which category of foods tends to be rich in monounsatured fats as well as low in saturated fats?
Which category of foods tends to be rich in monounsatured fats as well as low in saturated fats?
Nuts and oils are rich in monounsaturated fats and are low in saturated fats.
Sterols
STEROLS complex lipids with interconnected carbon rings with an oxygen, and a side chain consisting of carbons and hydrogens; a precursor for synthesis of steroid hormones
Chemically, sterols are complex lipids with four interconnected carbon rings with a hydrocarbon side chain.
CHOLESTEROL a sterol that is producedby the body and required for steroid production and cell membrane function
The most discussed sterol is cholesterol. A molecule with varied functions, cholesterol is a critical component of our cell membranes and is also needed as a precursor for the synthesis of bile acids, vitamin D, and steroid hormones such as estrogen and testosterone, but it does not provide any energy.

Cholesterol is synthesized in nearly every tissue in the body, but in particularly large quantities by the liver. Approximately 75% of the cholesterol in blood is made in our body, which provides all the cholesterol needed for body functions. Although a dietary source of cholesterol is not required, we consume cholesterol in animal foods, such as meats and dairy products. Indeed, the presence of cholesterol in cell membranes is one distinguishing characteristic between plant and animal cells, thus dietary cholesterol is found only in foods of animal origin. Plants synthesize other types of sterols (and the closely related stanols), but these types are poorly absorbed by the body and can actually interfere with and lower cholesterol absorption. For this reason, some spreads and other food products are fortified with plant sterols or stanols, to help lower cholesterol levels in the body. Chapter 7 further explores the relationship of cholesterol and other dietary fats on blood cholesterol.
Phospholipids
PHOSPHOLIPID a molecule that is both hydrophobic (water fearing) and hydrophilic (water loving) and is required to form cell membranes; lecithin, which can be found in egg yolks, liver, and some plant products, is a phospholipid
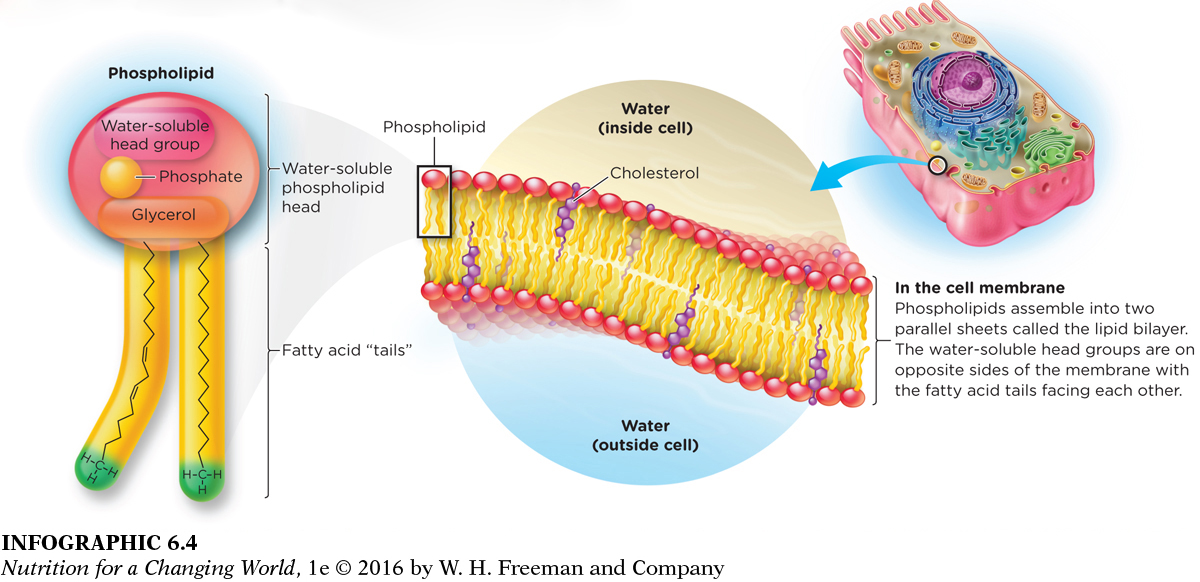
Phospholipids, the fourth most common category of lipids (after fatty acids, triglycerides, and sterols), are also the primary component of cell membranes and the structures that transport lipids in the blood. Because the body can produce phospholipids, they are not considered an essential nutrient. Phospholipids are similar to triglycerides in structure: they have a glycerol backbone, but with two rather than three attached fatty acids. Attached at the third position is a phosphate group and one of several water-
Question 6.4
 Why do the fatty acid “tails” face each other within the cell membrane?
Why do the fatty acid “tails” face each other within the cell membrane?
The fatty acid tails face each other within the cell membrane because they are not soluble in water (they are repelled by water).
LECITHIN the most abundant phospholipid in the body; frequently added to food products like salad dressings as an emulsifier
This two-
■ ■ ■
Dyerberg and Bang suspected that there might be something about the mix of fatty acids in the high-
The researchers scratched their heads. What was this mysterious fatty acid, and how was it protecting the Inuit from the number one cause of death in other parts of the world?
Even before Dyerberg and Bang began collecting blood samples from native Greenlanders, they suspected the then-