The uptake of gases from water is limited by diffusion
Almost 21 percent of Earth’s atmosphere is oxygen, but because we live on land we rarely think about the task of obtaining this required element. Aquatic organisms also require oxygen to support their metabolism, but obtaining a sufficient supply can be a problem for them because of the limited solubility of oxygen in water. The same is true of the CO2 required by aquatic plants for photosynthesis. Organisms obtain sufficient quantities of these necessary gases in the aquatic environment through several adaptations that we will examine in this section.
Carbon Dioxide
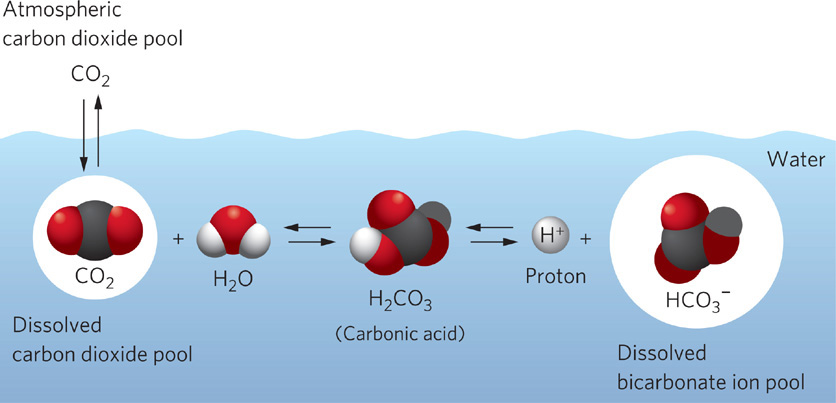
Getting enough CO2 for photosynthesis is a particular challenge for aquatic plants and algae. The solubility of CO2 in fresh water is about 0.0003 liters of gas per liter of water, which is 0.03 percent by volume, or about the same as its concentration in the atmosphere. The problem for aquatic plants is that CO2 diffuses slowly through water, and plants can use the gas close to the surface of their leaves faster than it arrives by diffusion. Fortunately, however, when CO2 dissolves in water, most of the molecules combine with water and are quickly converted to a compound called carbonic acid (H2CO3):
CO2 + H2O → H2CO3
As shown in Figure 2.14, carbonic acid can increase to high concentrations and provide a reservoir of carbon required for photosynthesis. Depending on the acidity of the water, carbonic acid molecules can release hydrogen ions (H+) to form either bicarbonate ions (HCO3−) or carbonate ions (CO32−):
H2CO3 → H+ + HCO3−
Bicarbonate ion (HCO3−) An anion formed by the dissociation of carbonic acid.
Carbonate ion (CO32−) An anion formed by the dissociation of carbonic acid.
Although some of the carbonate ions can combine with calcium ions to form calcium carbonate, as we saw in Figure 2.8, the bicarbonate ion is the most common form of inorganic carbon within the range of acidity that is typical of most aquatic habitats (pH values between 5 and 9). In addition, bicarbonate ions dissolve readily in water. The result, as shown in Figure 2.14, is a concentration of bicarbonate ions equivalent to 0.03 to 0.06 L of CO2 gas per liter of water (3 to 6 percent)—more than 100 times the concentration of CO2 in air. In short, this means that most of the CO2 that is dissolved into water is rapidly converted to bicarbonate ions and these bicarbonate ions provide an enormous reservoir of inorganic carbon in aquatic systems. Dissolved CO2 and bicarbonate ions are in a chemical equilibrium, which represents the balance achieved between H+ and HCO3 - on one hand, and CO2 and H2O on the other. When CO2 is removed from the water during photosynthesis some of the abundant bicarbonate ions combine with hydrogen ions to produce more CO2 and H2O:
46
H+ + HCO3− → CO2 + H2O
Aquatic plants and algae use CO2 and bicarbonate ions for photosynthesis. Although the bicarbonate ion is the most common form of inorganic carbon under moderate pH conditions, CO2 is the most common form under more acidic conditions, such as in bogs. Some species of plants and algae can only use CO2 for photosynthesis; these species live in aquatic habitats containing low pH. Species of plants and algae that live in moderate pH conditions, ranging from 5 to 9, can either directly uptake the bicarbonate ion and use it for photosynthesis or they can use adaptations that provide them with additional CO2. One way to do this is by secreting an enzyme into the water that is highly effective at converting bicarbonate ions into CO2, which can then be taken up by the organism for photosynthesis. Plants and algae can also obtain CO2 by secreting hydrogen ions into the surrounding water. This helps drive the chemical equilibrium in a direction that converts bicarbonate ions into CO2 that can then be taken up by the organism and used in photosynthesis.
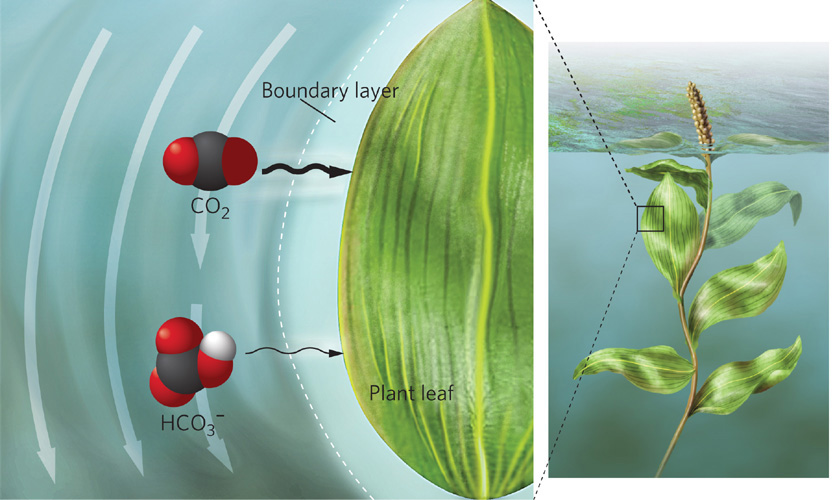
Boundary layer A region of unstirred air or water that surrounds the surface of an object.
Even when CO2 and bicarbonate ions are abundant in water, the slow rate at which these carbon sources diffuse through the water prevents organisms from getting access to them. Indeed, carbon dioxide diffuses through unstirred water about 10,000 times more slowly than through air, and diffusion of HCO3− is even slower because larger molecules diffuse at a slower rate. Compounding this slow rate of diffusion is the fact that every surface of an aquatic plant, alga, or microbe is surrounded by a boundary layer. A boundary layer is a region of unstirred air or water that surrounds the surface of an object. In the water, the boundary layer ranges from as little as 10 micrometers (10 μm, or 0.01 mm) for single-celled algae in turbulent waters to 500 μm (0.5 mm) for a large aquatic plant in stagnant water. As Figure 2.15 illustrates, because this boundary layer is composed of unstirred water, CO2 and HCO3− can be depleted within the boundary layer by uptake—especially in the region closest to the photosynthesizing organism—but the removed gases are slow to be replaced from the surrounding water. Without a boundary layer, the moving water in the surrounding environment would continually provide the plant with a supply of CO2 and HCO3−. So, despite the generally high concentration of bicarbonate ions in the water, photosynthesis may still be limited by carbon availability within the boundary layer.
47
Oxygen
Oxygen in the atmosphere has a concentration of 0.21 L per liter of air (21 percent by volume). In water, however, the maximum solubility of oxygen is 0.01 L per liter of water (1 percent), under the conditions of fresh water at 0°C. Oxygen’s low solubility in water can limit the metabolism of organisms in aquatic habitats. For marine mammals such as whales, this is not a problem because they obtain O2 from the air and they store copious amounts in their hemoglobin and myoglobin. For organisms that obtain O2 from the water, however, the problem of low O2 concentration is compounded by its slow diffusion in water, similar to that of CO2. Oxygen is in even shorter supply in waters that cannot support photosynthesis—and therefore do not receive the O2 it produces as a by-product—including in deep water that does not receive sunlight and in waterlogged sediments and soils. These habitats can become severely depleted of dissolved O2, making them challenging environments for animals and microbes that use aerobic respiration.
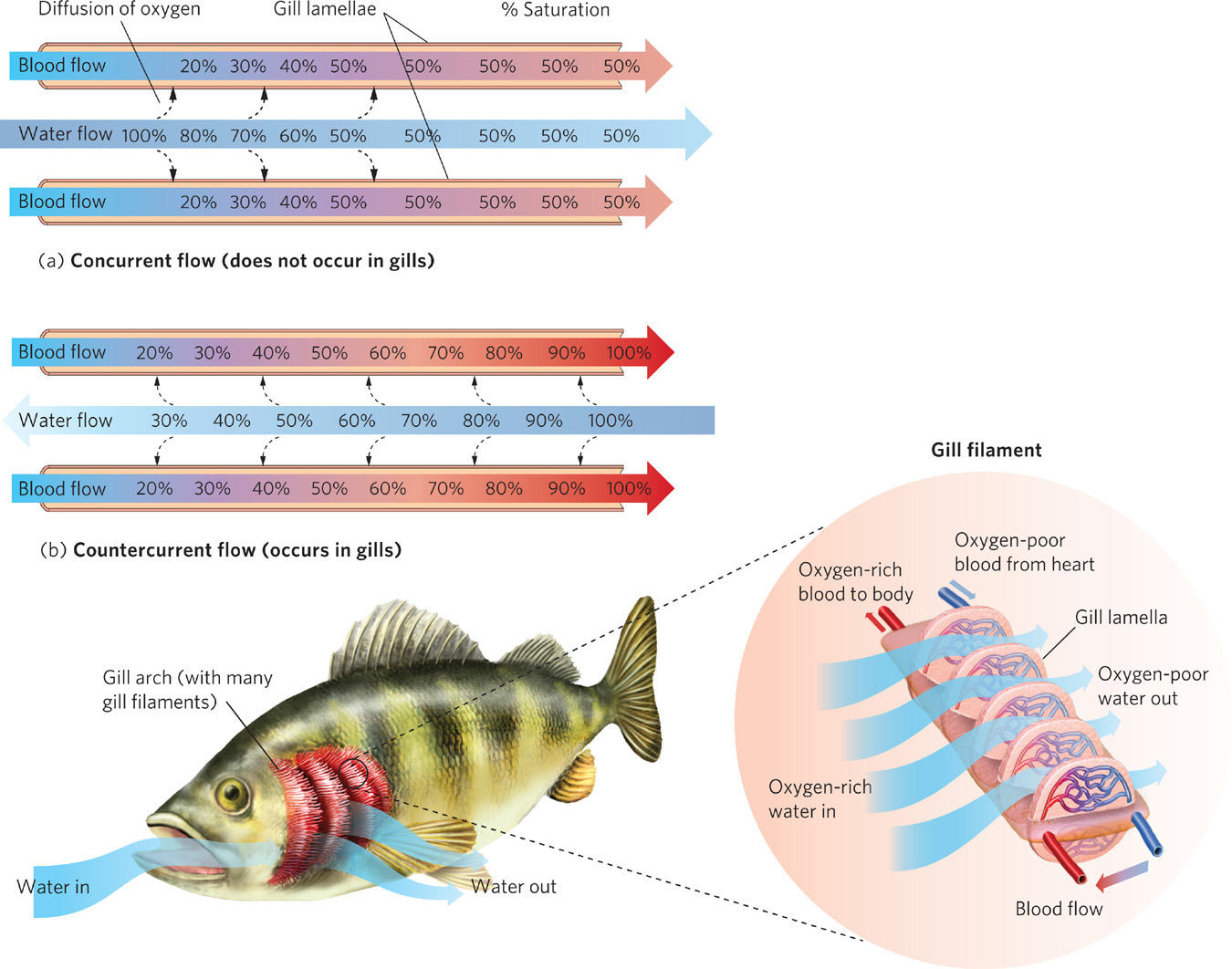
Countercurrent circulation Movement of two fluids in opposite directions on either side of a barrier through which heat or dissolved substances are exchanged.
One important adaptation that allows aquatic animals to deal with a limited amount of oxygen involves the direction of blood flow in the gills. Many aquatic animals have gills to extract oxygen from the water. When water passes over the gills, oxygen diffuses across the membranes of the gill cells and enters the capillaries, which are part of the bloodstream. The key to extracting the most oxygen from the water lies in the use of countercurrent circulation. In countercurrent circulation, two fluids move in opposite directions on either side of a barrier and heat or materials are exchanged. In contrast, concurrent circulation involves two fluids movingin the same direction on either side of a barrier and heat or materials are exchanged. As illustrated in Figure 2.16, if blood and water were to flow in the same direction the concentration of oxygen would quickly come to an intermediate equilibrium. After this region of contact, there is no net movement of oxygen across the membrane. In contrast, when blood and water flow in opposite directions the concentration of oxygen in the water exceeds the concentration in the blood throughout most of the region of contact. This happens because even as the capillaries begin to develop high concentrations of oxygen, the adjacent water still has a higher concentration of oxygen. As a result, the oxygen continues to diffuse into the gill capillaries. Thus, countercurrent blood flow in animal gills allows much more oxygen to move from the water to the gills.
Concurrent circulation Movement of two fluids in the same direction on either side of a barrier through which heat or dissolved substances are exchanged.
Species of animals that live in habitats with low amounts of oxygen have evolved a number of additional adaptations. In the deep oceans, many organisms have very low rates of activity, thereby reducing their need for oxygen. Many species of zooplankton, a group of tiny crustaceans, can increase the amount of hemoglobin in their bodies to the point that their normally transparent bodies turn red. Other animals, such as tadpoles and fish that live in oxygen-depleted swamps, swim to the surface and take gulps of air. Many tadpoles can use this air because they possess primitive lungs in addition to gills. Fish store this air in a swim bladder from which they extract the oxygen into their bloodstream.
48
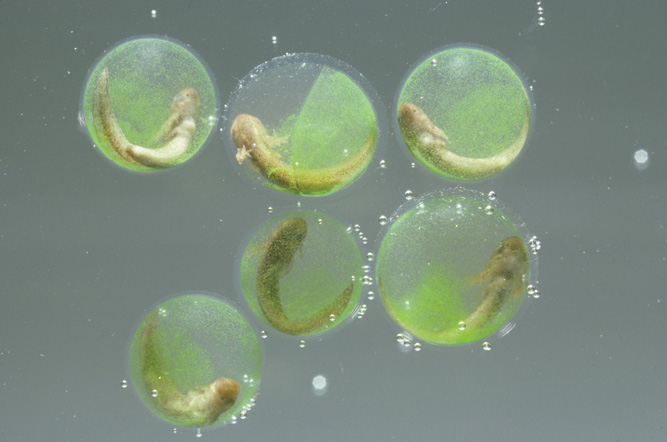
One of the most surprising animal adaptations for obtaining oxygen was recently discovered in a species of North American salamander. For more than a century it was known that eggs of the spotted salamander, which are usually attached to sticks that are submerged in water, have a mutualistic relationship with a species of algae (Oophila amblystomatis). The algae obtain a place to live and photosynthesize while the developing embryo obtains oxygen from the photosynthesizing algae (Figure 2.17). This oxygen benefit is important because it allows the salamander embryos to have a higher survival rate and to hatch earlier and larger. In 2011, however, scientists reported that this relationship was much closer than they had appreciated. The algae not only live in the fluid of the egg surrounding the embryo, but also move into the embryo, positioning themselves in between the developing embryo’s cells. This was the first discovery of an algae living within the tissues of a vertebrate animal.
Anaerobic Without oxygen. Also known as Anoxic.
When an environment becomes completely devoid of oxygen, it is referred to as anaerobic or anoxic. Anaerobic conditions pose problems for terrestrial plants rooted in waterlogged soils, such as the many species of mangrove trees that live along coastal mudflats. The roots of these trees need oxygen for respiration, so the plants have evolved special air-filled tissues extending from the roots that rise above the waterlogged soils and exchange gases directly with the atmosphere (see Figure 2.13).
49
Many microbes are able to live in environments without oxygen because they use anaerobic respiration. A common product of anaerobic respiration by bacteria living in anoxic soils is hydrogen sulfide gas (H2S). This gas is the cause of the rotten-egg smell that occurs when soils that are saturated with water become anaerobic.