When Oxygen is Scarce
FERMENTATION A series of chemical reactions that takes place in the absence of oxygen and converts some of the energy stored in food into ATP. Fermentation produces far less ATP than does aerobic respiration.
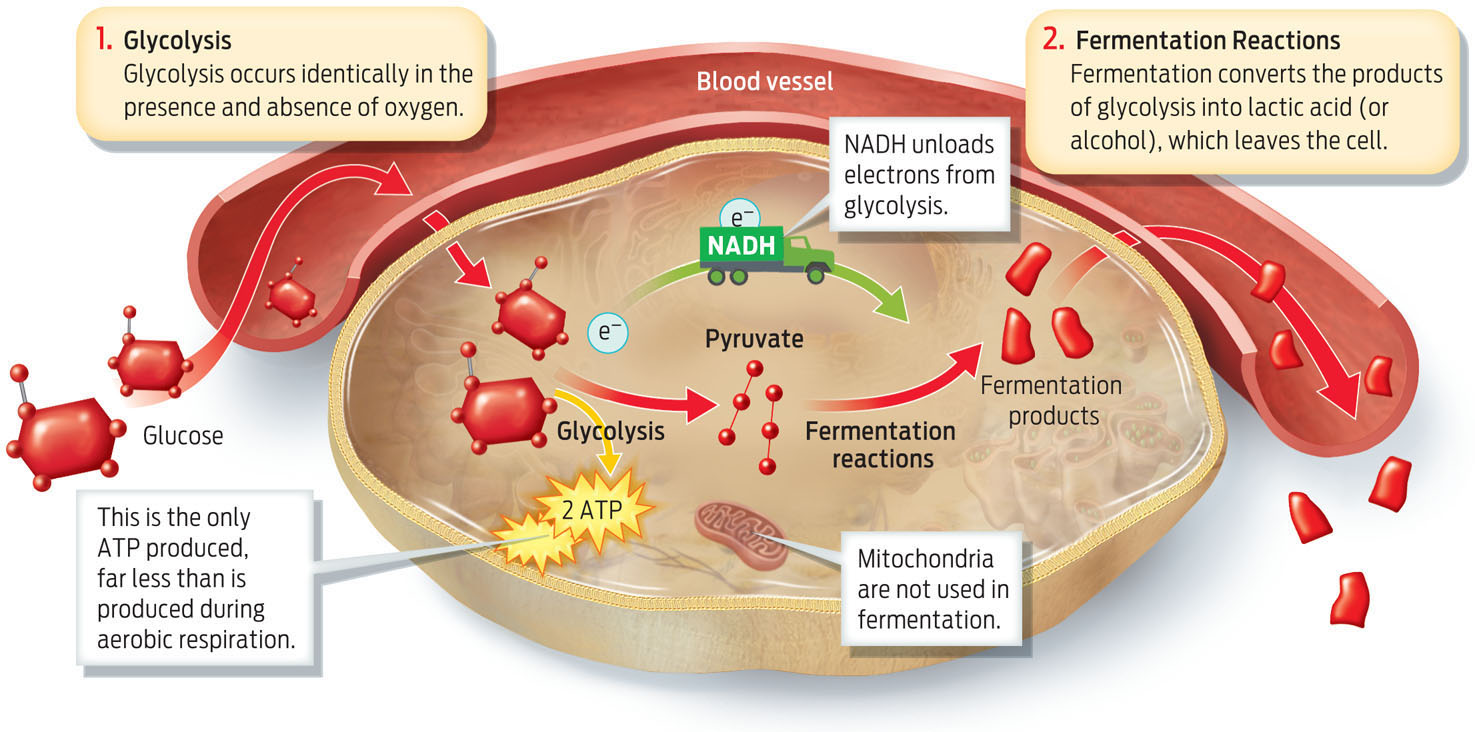
In order to keep performing aerobic respiration, cells need adequate oxygen. If the rate at which cells consume oxygen exceeds the rate at which they take it in when we breathe, aerobic respiration comes to a halt, because the electron transport chain has no oxygen to which it can deliver electrons. Without oxygen, glycolysis still occurs, but its products are shunted into a different process, fermentation, which takes place in the cell’s cytoplasm (as opposed to the mitochondria).
Fermentation does not actually produce any more ATP beyond what is produced by glycolysis. So why do cells do it? In essence, it’s a way to keep glycolysis running. As the cell carries out glycolysis in the absence of O2, glucose will continue to give up electrons to NAD+ as it is converted to pyruvate. However, in the absence of O2, NADH cannot unload its electrons to the electron transport chain, and therefore there is no NAD+ available to pick up electrons from glucose (since all the NAD will be in the NADH form). Soon glycolysis will grind to a halt unless there is some way for the cell to regenerate NAD+. This is the purpose of fermentation: during fermentation, NADH unloads its electrons onto pyruvate, thus regenerating NAD+, which allows glycolysis to keep running and producing small amounts of ATP.
Because fermentation bypasses both the citric acid cycle and the electron transport chain, much less ATP is produced—only about 2 molecules of ATP from each molecule of glucose compared to about 36 ATP produced by aerobic respiration. Instead of carbon dioxide, fermentation produces lactic acid (INFOGRAPHIC 6.9) .
Glycolysis occurs whether or not oxygen is present. In the absence of oxygen, fermentation reactions follow glycolysis. Fermentation occurs in the cytoplasm and converts the products of glycolysis into lactic acid (or alcohol in some organisms). The only ATP produced is the small amount produced during glycolysis.
128
In humans, fermentation takes place primarily during bursts of energy-intensive tasks, such as sprinting or power weight-lifting. It is, in essence, a backup plan for times when oxygen isn’t available. The panting you experience on a treadmill is your body’s way of trying to obtain more oxygen. (The idea that lactic acid buildup is the cause of muscle cramping is a myth.)
But for many organisms, like certain fungi and bacteria, fermentation is the main way of obtaining energy. In some of these organisms, fermentation produces alcohol rather than lactic acid as a by-product. Humans take advantage of these fermentation reactions when they make alcoholic beverages. Brewer’s yeast, for example, is a fungus that ferments sugar in the absence of oxygen, producing alcohol as a result. Humans use brewer’s yeast to make beer and wine.
Since fermentation does not break glucose down as completely as does aerobic respiration, there is still quite a bit of carbohydrate energy left in such beverages as beer and wine, about 7 Calories per gram—which explains why most weight-loss diets eliminate alcohol.

Even during aerobic respiration, however, our bodies don’t convert every Calorie in food into ATP. The chemical processes aren’t 100% efficient, so some energy is always released as heat, which keeps your body warm.
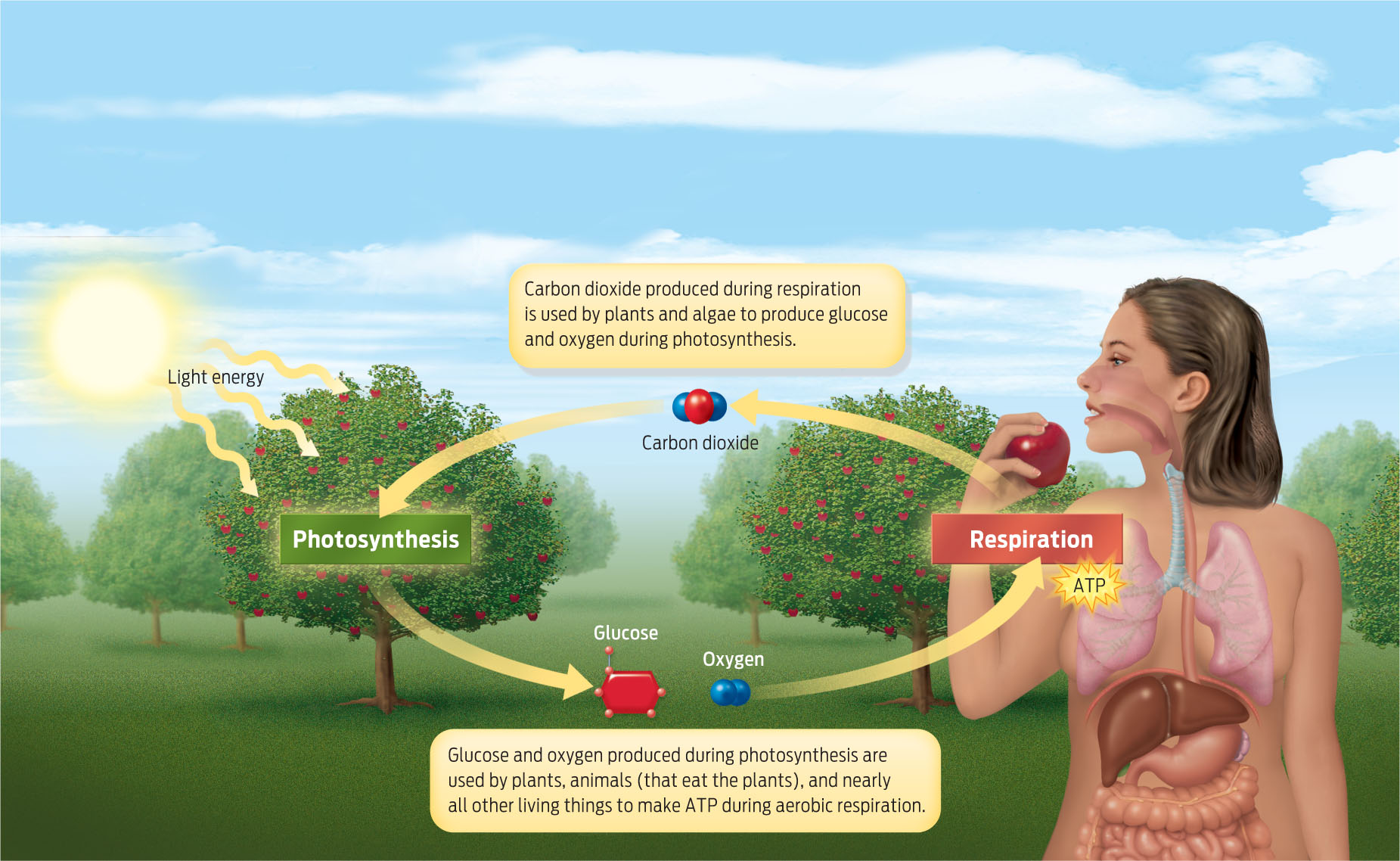
It’s important to remember that aerobic respiration does not create energy—it only extracts it from food and converts it into ATP. All the food we eat—whether burger, chicken leg, or Caesar salad—originally gets its energy from the sun, by way of photosynthesis (see Chapter 5). Photosynthesizers such as plants and algae capture the energy of sunlight and convert it into chemical energy stored in sugar. We then eat this sugar (or eat animals that have eaten this sugar), and that stored energy becomes available to us. Plants benefit from the relationship, too: plants use our carbon dioxide waste as raw material for making sugar during photosynthesis. In this way, photosynthesis and respiration form a continual cycle, with the outputs of one process serving as the inputs of the other (INFOGRAPHIC 6.10) .
Photosynthesis and respiration form a continuous cycle, with the outputs of one process serving as the inputs of the other.
129