FOLLOW THE WATER
In their search for extraterrestrial life, astrobiologists often use this rule of thumb: “Follow the water.” Water is viewed as a proxy for life because it is so crucial to life on Earth. Water makes up more than 75% of a cell’s weight. All of life’s chemical reactions take place in water, and many living things can survive only a few days without it.
POLAR MOLECULE A molecule in which electrons are not shared equally between atoms, causing a partial negative charge at one end and a partial positive charge at the other; for example, water.
HYDROGEN BOND A weak electrical attraction between a partially positive hydrogen atom and an atom with a partial negative charge.
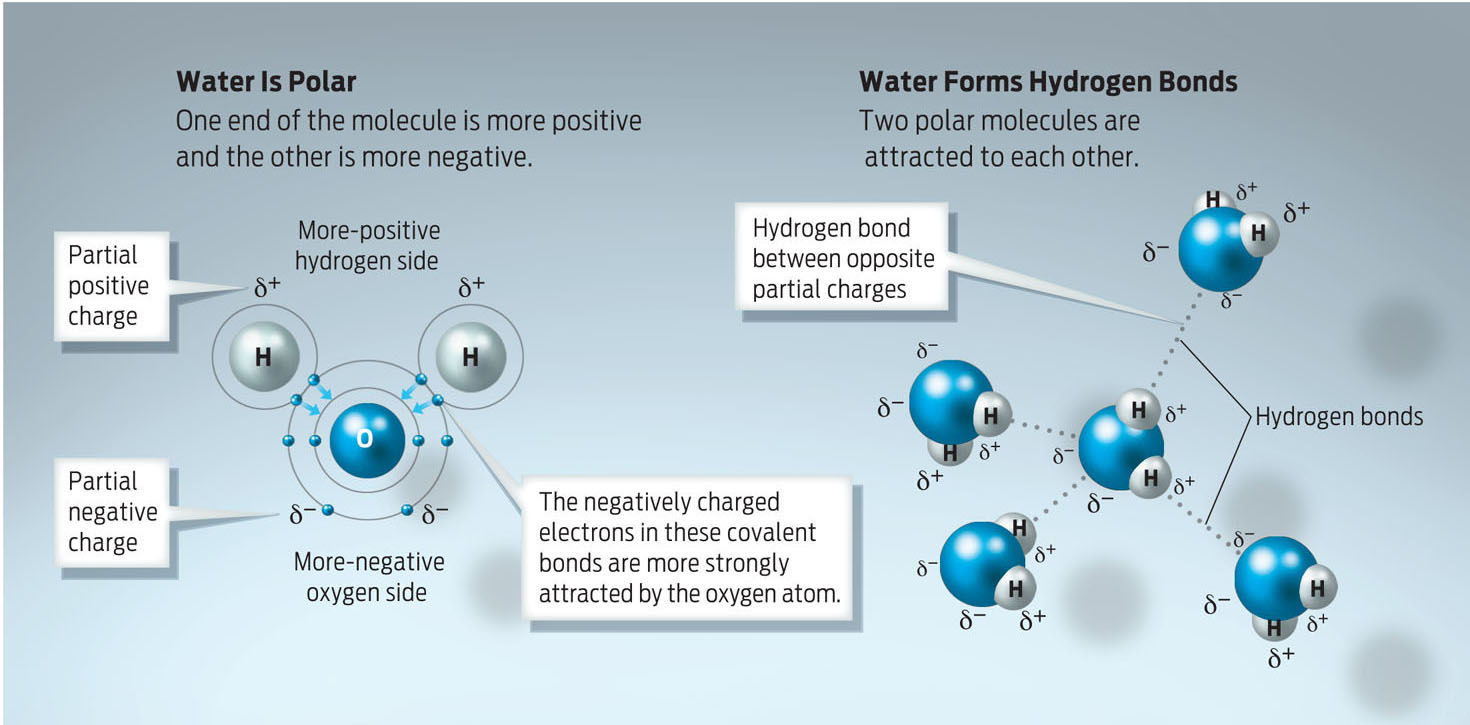
A water molecule–H2O–is shaped like Mickey Mouse’s head (the oxygen atom is the face, and the two hydrogens are the ears). Many of water’s life-conducive properties are a function of this simple shape. Water is a polar molecule meaning that the electrons in the bonds between oxygen and hydrogen in water are shared unequally; the oxygen atom has a stronger pull on electrons than the hydrogen atoms do, giving water a partial negative charge on one side and a partial positive charge on the other. When water molecules are near one another, the partial negative and partial positive charges of water molecules attract one another, forming weak electrical interactions known as hydrogen bonds (INFOGRAPHIC 2.6).
Water is a polar molecule because electrons are not shared equally between the oxygen and the hydrogen atoms in each of its polar covalent bonds. Electrons are pulled closer to the oxygen atom than to the hydrogen atoms, creating a slightly negative oxygen atom and a slightly positive hydrogen atom. When many water molecules are near one another, the partially positive hydrogen atoms of some molecules are attracted to the partially negative oxygen atoms of neighboring water molecules. These attractions are hydrogen bonds, weak electrical attractions.
COHESION Water molecules sticking to water molecules through hydrogen bonding.
ADHESION Water molecules sticking to other surfaces through hydrogen bonding.
Hydrogen bonds are crucial for many of water’s life-sustaining properties. First, they make water “sticky,” acting as a kind of glue holding water molecules together. The stickiness of water allows water molecules to cling to one another (cohesion) or to a surface (adhesion). You can see evidence of water’s stickiness wherever you look: a drop of water clinging to a leaf despite the downward pull of gravity, or an insect able to walk on the surface of a pond.
Compared to other molecules its size, water has a large liquid range—freezing at 0°C (32°F) and boiling at 100°C (212°F). That’s because water molecules can absorb a lot of energy before they get hot and vaporize (that is, turn into a gas)—again because of their tenacious hydrogen bonds. Water’s liquid range can be extended even further: add salt and you can lower the freezing point to –46°C (–50°F); increase the pressure and you can bump up the boiling point to over 343°C (650°F). It’s because there is so much salt in seawater that most oceans don’t freeze in winter.
Unlike most substances on Earth, water has the unusual property of being less dense as a solid than as a liquid: ice floats. When water freezes, the water molecules form an ordered array, with individual water molecules spaced at equal and fixed “arm’s length” from one another. This increased separation between water molecules decreases the density of ice, allowing ice to float on water. And because it does, fish can live beneath frozen lakes in winter and not turn into ice cubes (INFOGRAPHIC 2.7).
SOLVENT A substance in which other substances can dissolve; for example, water.
SOLUTE A dissolved substance.
Perhaps the most important life-conducive property of water is that it is a universal solvent, capable of dissolving just about any substance—even gold. Water transports all of life’s dissolved molecules, or solutes, from place to place-whether through a cell, a body, or an ecosystem. Life, in essence, is a water-based solution. In fact, many biological molecules, like proteins and DNA, have the specific shapes they do only because of the surrounding water with which they interact.
SOLUTION The mixture of solute and solvent.
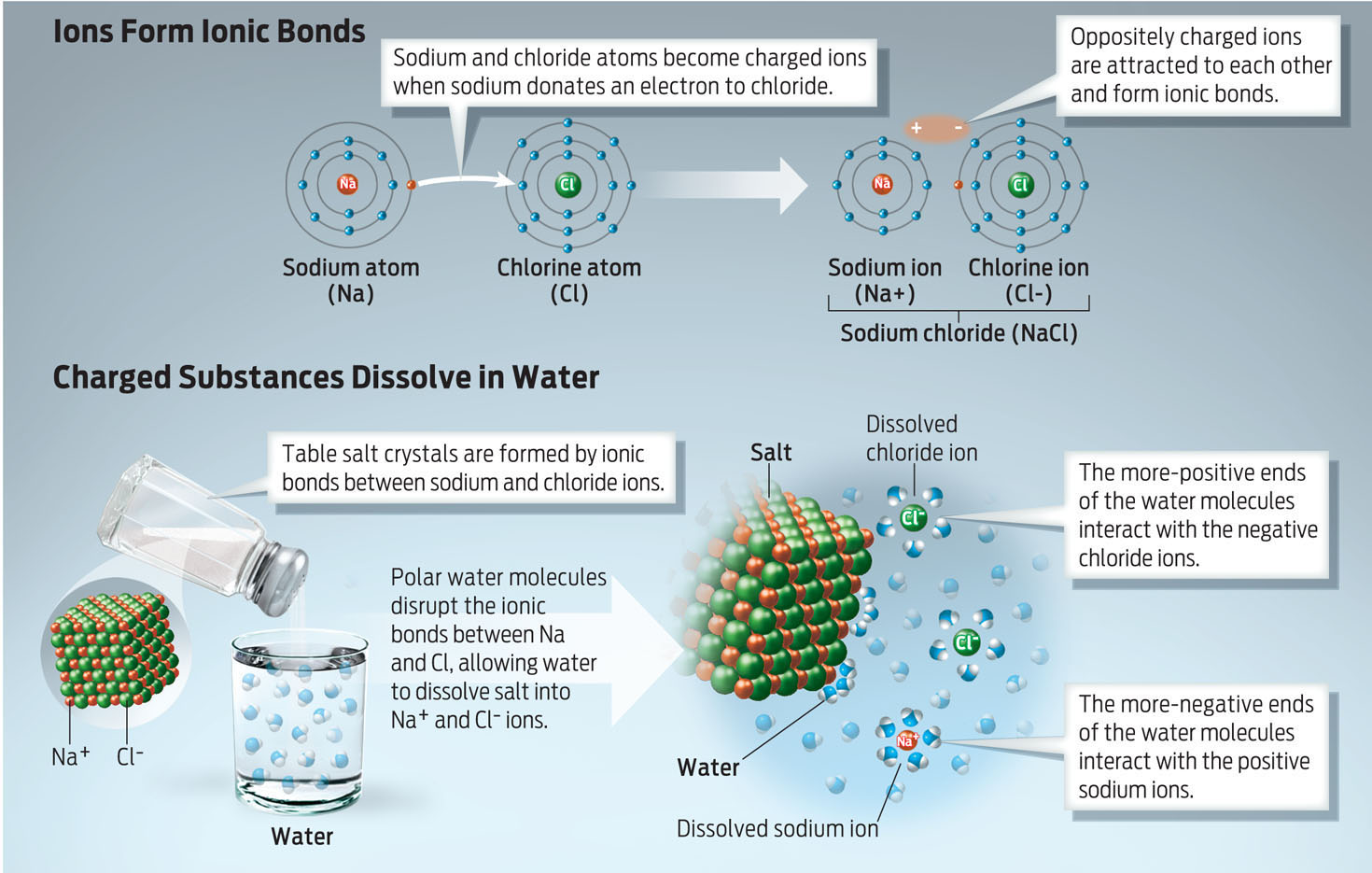
IONIC BOND A strong electrical attraction between oppositely charged ions formed by the transfer of one or more electrons from one atom to another.
ION An electrically charged atom, the charge resulting from the loss or gain of electrons.
Water is an excellent solvent for other polar molecules with partial charges and for substances, like salt, that contain ionic bonds. Ionic bonds form between atoms that have opposite electrical charges (positive and negative). Such charged atoms, or ions, form when one atom loses a negatively charged electron (becoming a positively charged ion) and another atom gains that electron (becoming a negatively charged ion). Ionic bonds are the strong bonds formed between these oppositely charged ions. By surrounding each charged ion, water dissolves the bond between them (INFOGRAPHIC 2.8).
Because water molecules have partial charges, they can interact with charged ions and other hydrophilic molecules, allowing water to coat and then dissolve these solutes.
When astrobiologists speak about the importance of water for life, they make an important qualification: liquid water. Frozen water is found throughout the universe; there are abundant quantities on Mars, for example. But only on Earth does water exist primarily in its liquid form at room temperature. “Liquid water is the key requirement in the search for life,” says McKay. “The other worlds of the solar system have enough light, enough carbon, and enough of the other key elements for life. Water in the liquid form is rare.”
Though liquid water is not present on the surface of Mars today, scientists suspect that liquid water—lots of it—once covered the planet. Clues to this ancient water can be seen all over the surface of the planet, which in many places is carved out like sections of the Grand Canyon. Phoenix Lander also found telltale signs of liquid water’s past on the surface of Mars in the form of salt deposits like those you can see when seawater evaporates. “The case for water, we could say, is tight,” says McKay.
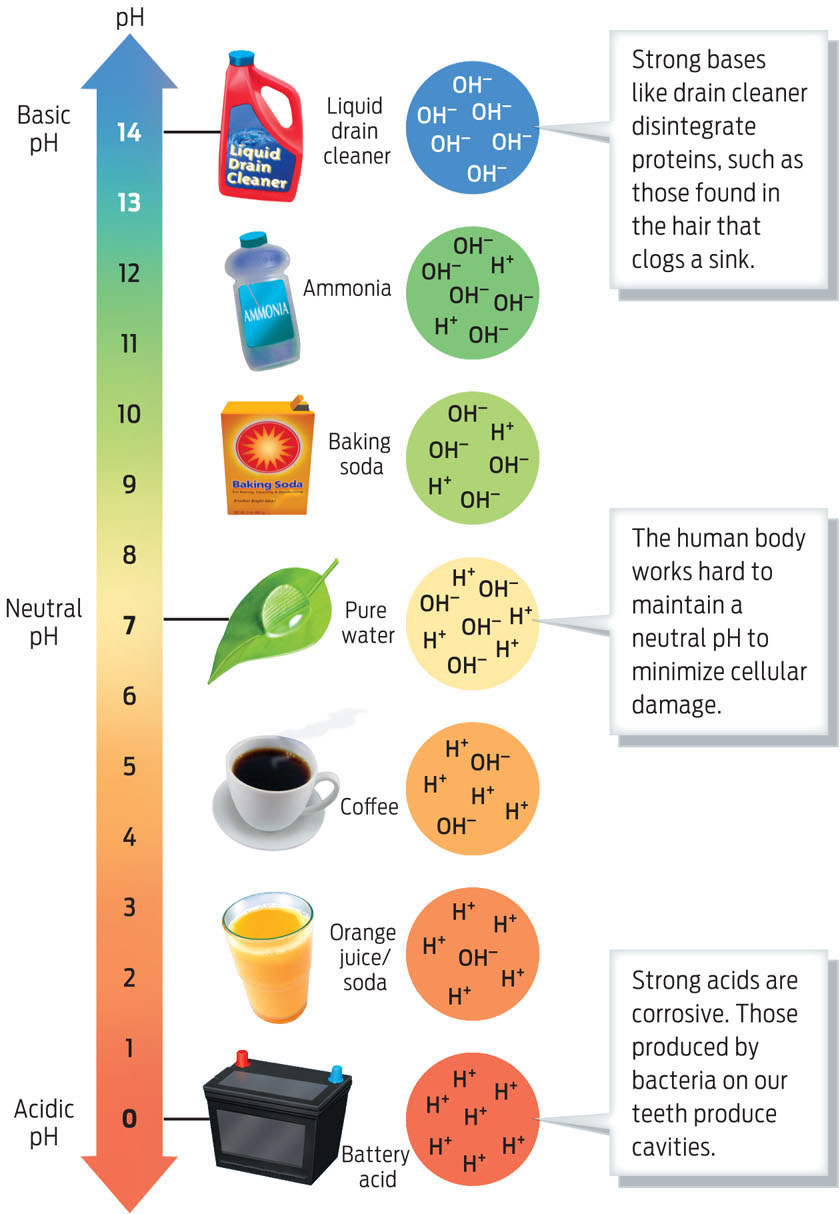
pH A measure of the concentration of H+ in a solution.
Where all this water went, no one knows. But some believe that liquid water may still lurk beneath the surface of the planet, and may even bubble to the surface periodically, as is suggested by photographs of apparent water flows taken in 2004 and 2005 by NASA’s Mars Global Surveyor satellite.
ACID A substance that increases the hydrogen ion concentration of solutions, making them more acidic.
If there is water within Mars, would it have the properties of Earth water and therefore support life? Depending on what’s dissolved in it, water can have a wide range of characteristics–from caustic drain cleaner to calming chamomile tea. The different chemical properties of water-based solutions reflect their pH, the concentration of hydrogen ions (H+) in a solution, defined as ranging from 0 to 14. In every water-based solution, water molecules (H2O) split briefly into separate hydrogen (H+) and hydroxide (OH−) ions. In pure water, the number of separated H+ ions is by definition exactly equal to the number of separated OH− ions, and the pH is therefore 7, or neutral–the dead center of the 0 to 14 scale. Acidic solutions, or acids, have a higher concentration of hydrogen ions (H+) and a pH closer to 0. When acids are added to water, they increase the concentration of hydrogen ions and make the solution more acidic. Basic solutions, or bases, on the other hand, have a lower concentration of H+ ions and a pH closer to 14. Bases remove H+ ions from a solution, thereby increasing the proportion of OH− ions.
BASE A substance that reduces the hydrogen ion concentration of solutions, making them more basic.
Strong acids and bases are highly reactive with other substances, which makes them destructive to the molecules in a cell. Also, many biochemical reactions take place only at a certain pH. Living things are thus extremely sensitive to changes in pH, and most function best when their pH stays within a specific range. The pH of human blood is about 7.4. If that pH were to fall even slightly, to 7, our biochemistry would malfunction and we would die. Phoenix Lander calculated the pH of Mars as 7.7—mild enough to grow asparagus, as the mission’s chief chemist put it (INFOGRAPHIC 2.9).
The pH of a solution is a measure of the concentration of hydrogen ions (H+) in it. Solutions with a low concentration of H+ ions have a basic pH (greater than pH 7). Solutions with a high concentration of H+ ions have an acidic pH (a pH of less than 7). Both acids and bases can be damaging because they are highly reactive with other substances. A neutral solution has a pH of 7.