VIRAL TIME CAPSULE
In 1995, Jeffery Taubenberger, a virologist at the U.S. Armed Forces Institute of Pathology, became interested in the mystery of the 1918 flu, and wondered if advances in molecular biology might help him solve it. He realized that he could use the polymerase chain reaction (PCR; see Chapter 7) to reconstruct the viral genome—if only he had access to some original tissue samples from victims. He knew that the army had routinely saved tissue samples from soldiers killed during wartime and decided to check the institute’s archives. Right away, he was able to locate tissues from several U.S. servicemen who had died of flu in 1918. Over the next several months, Taubenberger and his colleagues used PCR to recover viral RNA from these samples and then were able to sequence a few of the virus’s genes. But the results were incomplete, and the team eventually ran out of tissue samples from which to obtain virus. Taubenberger published these preliminary results in 1997 in the journal Science.
Later that year, Johan Hultin, a Swedish pathologist and physician, came across Taubenberger’s paper and realized he had a solution to the problem of finding more tissue samples. Back in the 1950s, while doing research for his dissertation, Hultin had discovered a mass grave of Spanish flu victims outside a remote village in Alaska. The village had been hit especially hard from the pandemic–85% of villagers had died from flu. Because the victims were buried in permafrost, their tissues might still be intact, he reasoned. Hultin wrote to Taubenberger and offered to take him to the site.
With permission from village elders, the scientists dug into the permafrost and exhumed dozens of bodies. Most were not much more than bone, but the body of one obese woman was still relatively intact, preserved by layers of insulating fat. From her tissues, the scientists were able to obtain enough viral RNA to resurrect the complete 1918 flu virus sequence–a landmark breakthrough. From this sequence, published in 2005, researchers were able to determine the identity of the virus and analyze its genetic relationship to other known strains of flu virus. But that’s not all. Knowing the sequence of all eight of the virus’s genes allowed researchers to begin asking which genes were responsible for the virus’s extreme virulence.
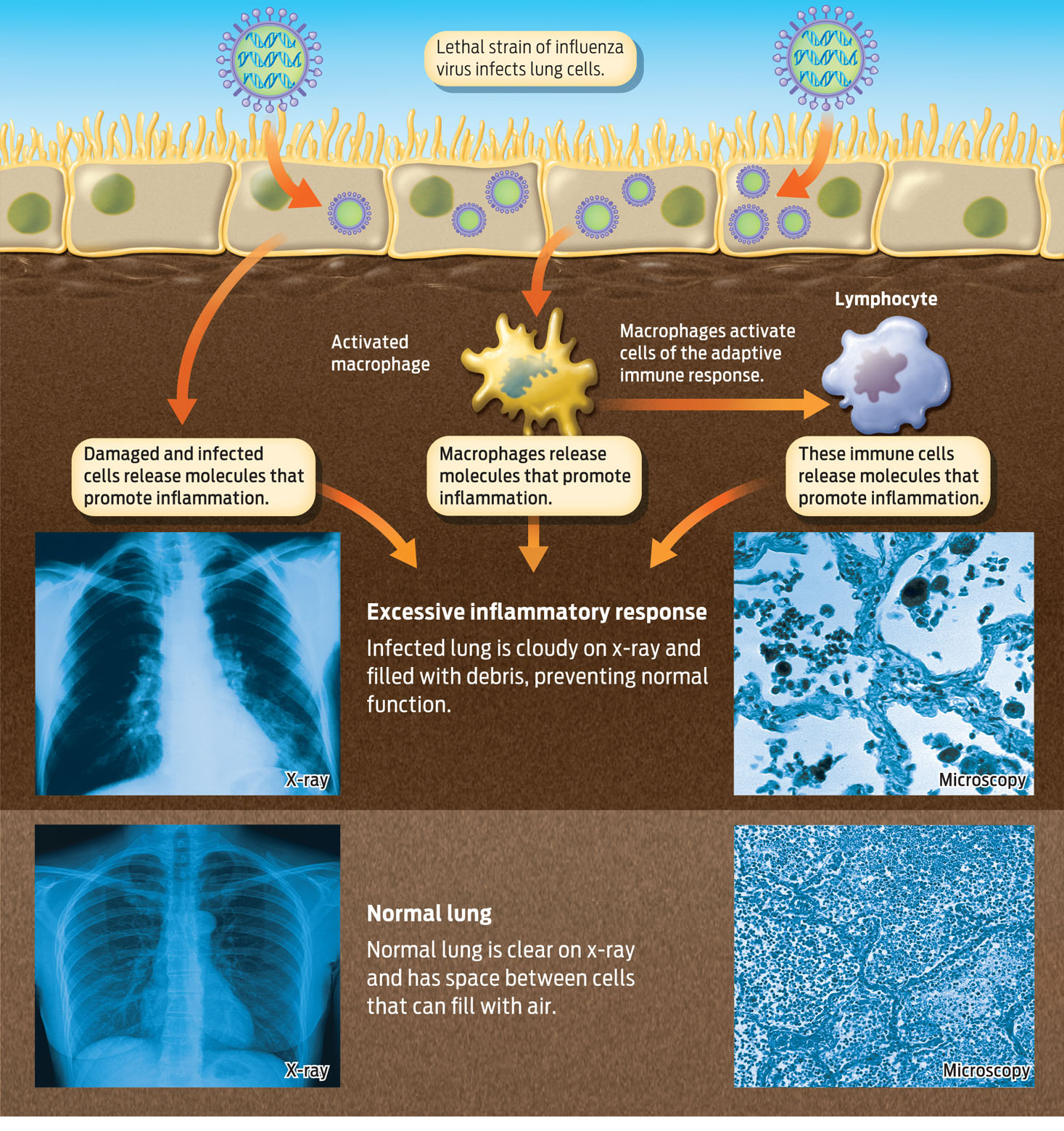
A few years later, in 2008, Yoshihiro Kawaoka, at the University of Wisconsin, Madison, and his colleagues used this information to determine what made the 1918 virus so deadly: four genes with alleles specific to the 1918 virus allowed flu virus to penetrate deep into the lungs and cause infection there. While less lethal flu viruses replicate primarily in the upper respiratory tract, the versions of genes in the 1918 virus allowed it to cruise past the mouth, nose, and throat and to invade the lungs. Replication of the virus in the lungs triggered a massive inflammatory response.
The inflammatory response is generally a good thing: it is a very effective way to contain and destroy invaders. But the balance between defense and destruction is delicate. In unusual cases, the inflammatory response can go into overdrive and destroy the very organ it is trying to save. This is what researchers now think happened with the 1918 flu. In the face of such an aggressive flu virus, the inflammatory response of victims was so massive that it damaged the very tissue it was supposed to protect. As fluids leaked out of blood vessels, they impaired oxygen uptake in the lungs; and as phagocytes produced chemicals to kill pathogens, those same chemicals destroyed lung tissue. What killed many people was not the virus itself but the overly aggressive inflammatory response mounted against it. This aggressive immune response also explains why victims with the strongest immune systems–people age 20 to 40–were the ones who suffered most (INFOGRAPHIC 31.5).
Other patients likely died from secondary infections occurring after flu infection. Because influenza weakened the defenses of the respiratory tract, other pathogens, such as bacteria, had unimpeded entry into the lungs. And because antibiotics were not yet discovered (see Chapter 3), doctors had no way of treating these infections. Bacterial pneumonia was responsible for at least half of all deaths during the 1918 pandemic, according to Taubenberger.