The heat of nuclear reactions can be harnessed to produce electricity.
In some ways, electricity generated using nuclear energy is no different than other forms of thermoelectric power (those that use heat to produce electricity). Just like power plants that run on oil or coal, nuclear plants use heat to boil water and produce steam, which is then used to generate electricity. The difference, really, is in where that heat comes from. Coal and oil plants create it by burning fossil fuels. In the thermonuclear production of electricity, heat is produced through a controlled nuclear reaction—usually a nuclear fission reaction.
nuclear energy
Energy released when an atom is split (fission) or combines with another to form a new atom (fusion).
nuclear fission
A nuclear reaction that occurs when a neutron strikes the nucleus of an atom and breaks it into two or more parts.


KEY CONCEPT 22.1
Radioactive isotopes are the starting material for the thermonuclear production of electricity.
Nuclear fission reactions are those that result in the splitting of an atom. Specifically, these reactions involve a special type of atom known as a radioactive isotope. Some elements can exist in two or more forms; each form has the same number of protons and electrons but a different number of neutrons and, hence, a different atomic mass; these different versions of the atom are called isotopes. INFOGRAPHIC 22.1
isotopes
Atoms that have different numbers of neutrons in their nucleus but the same number of protons.
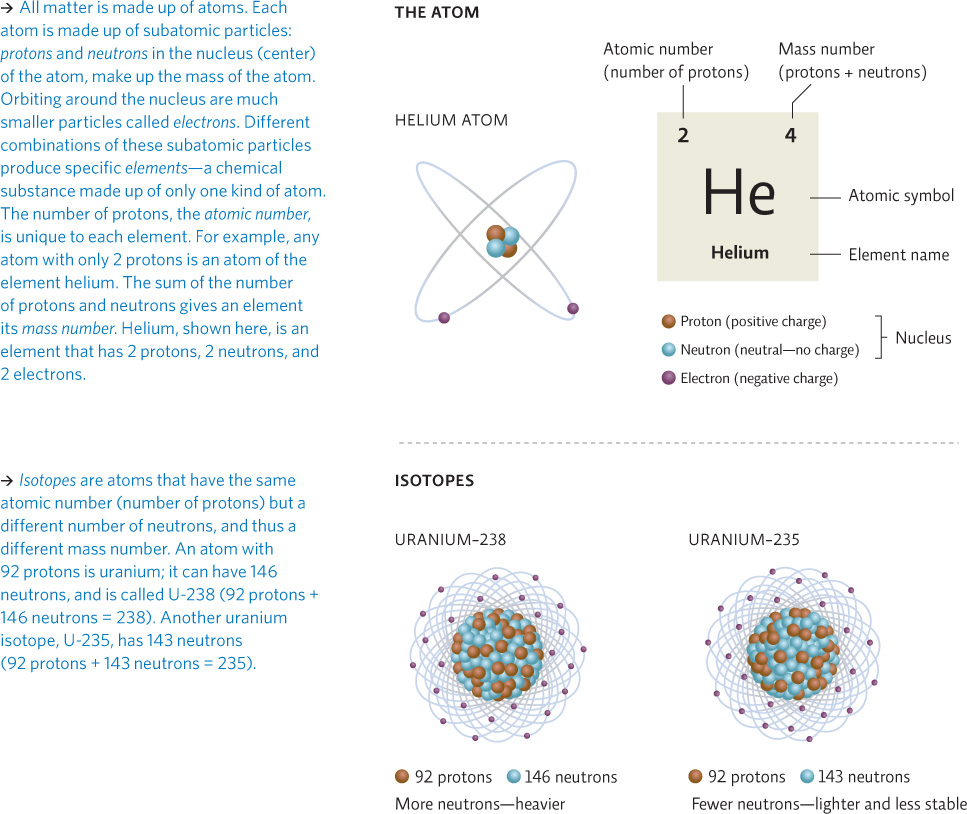

Uranium also exists as uranium-233. How many protons and neutrons does it have? Do you think it is more or less stable than U-235? Explain.
U-233 has 92 protons and 141 neutrons. U-233 is probably less stable than U-235 because it is lighter. [U-233 has a half-life of 160,000 years; U-235 has a half-life of 700,000,000 years.]
KEY CONCEPT 22.2
Radioactive decay is measured in terms of a half-life and can vary in duration for different isotopes, from milliseconds to billions of years.
Most isotopes are stable, meaning they do not spontaneously lose protons or neutrons. But some are radioactive: They emit subatomic particles and heat energy (radiation) in a process known as radioactive decay. Radioactive decay is measured in half-lives. An isotope’s radioactive half-life is the amount of time it takes for half of the radioactive material in question to decay to a new form. So after one half-life, 50% of the material will decay; in the next half-life, 50% of what’s left (or 25% of the original amount) will then decay. After 10 half-lives, just 0.1% of the original radioactive material is left. INFOGRAPHIC 22.2
radioactive
Atoms that spontaneously emit subatomic particles and/or energy.
radioactive half-life
The time it takes for half of the radioactive isotopes in a sample to decay to a new form.
The rate of decay for a given radioactive isotope is predictable and expressed as a half-life—the amount of time it takes for half of the original radioactive material (parent) to decay to the new daughter material (a new isotope, or even a new atom if protons are lost). Radioactive isotopes and their daughter radioactive isotopes continue to decay until they form a stable isotope that no longer loses particles. For instance, U-238 decays initially to thorium-234, which itself will decay over time. The entire decay sequence of U-238 includes progression through at least 13 isotopes (each step with its own half-life that ranges from milliseconds to thousands of years) until the final isotope decays into lead-206, a stable atom.
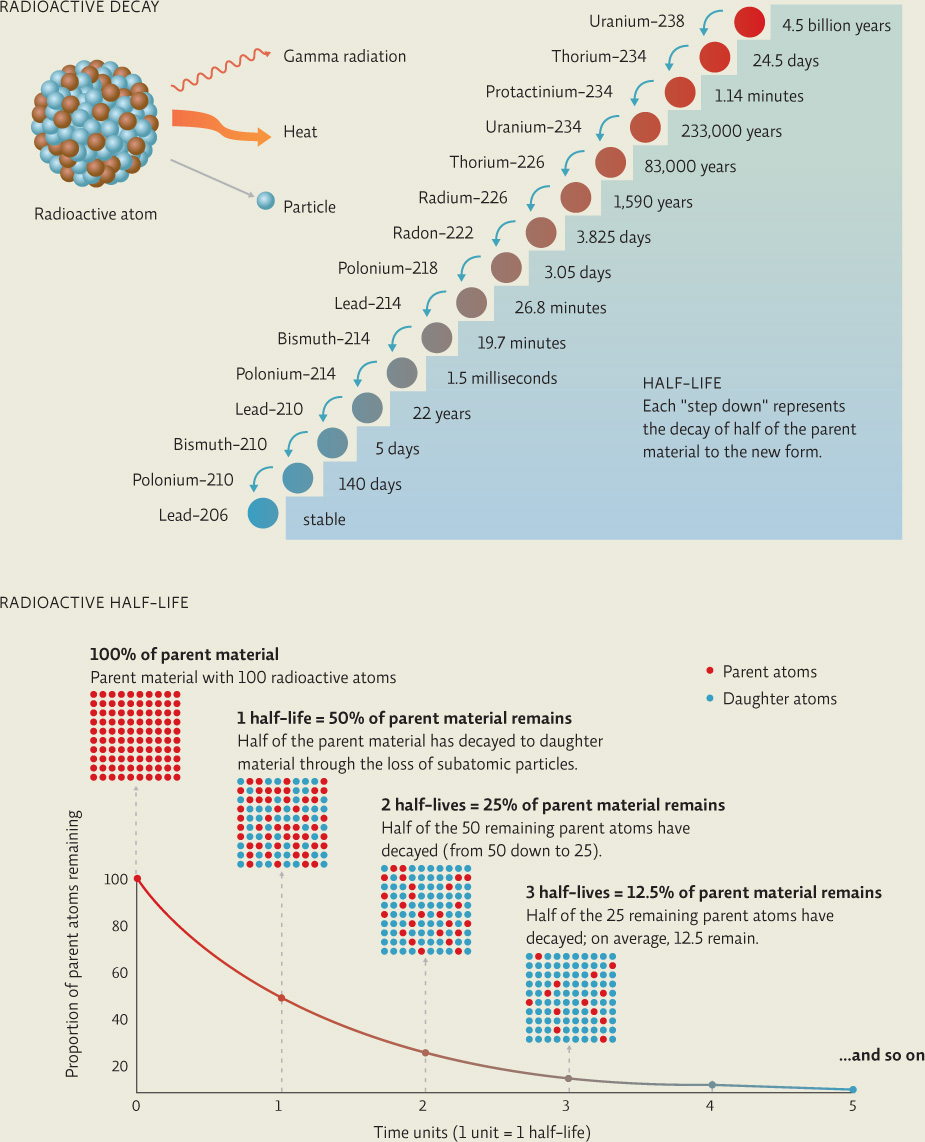

What percentage of the parent material will be left after 5 half-lives?
3.13%
KEY CONCEPT 22.3
The production of nuclear fuel involves mining and several processing steps, all of which generate hazardous waste.
Most nuclear reactors use uranium, which has several isotopes. Uranium-238 (U-238) is the most stable and is the most abundant form of uranium; it makes up roughly 99% of Earth’s total supply. But it’s U-235, the most reactive form, that is mined from Earth, processed into nuclear fuel (which, like all other mining work, involves both safety and environmental hazards), and packed into the fuel rods that are used in facilities like Fukushima. Estimates for supplies of uranium range from 80 to 200 years, but the worry that we would run out of nuclear fuel is less of an issue than it is with fossil fuels; other isotopes can also be used. INFOGRAPHIC 22.3
fuel rods
Hollow metal cylinders filled with uranium fuel pellets for use in fission reactors.
Uranium ore (rock that contains uranium) is mined and goes through many stages of processing to produce fuel suitable for a nuclear reactor. The process creates hazardous waste at every step.
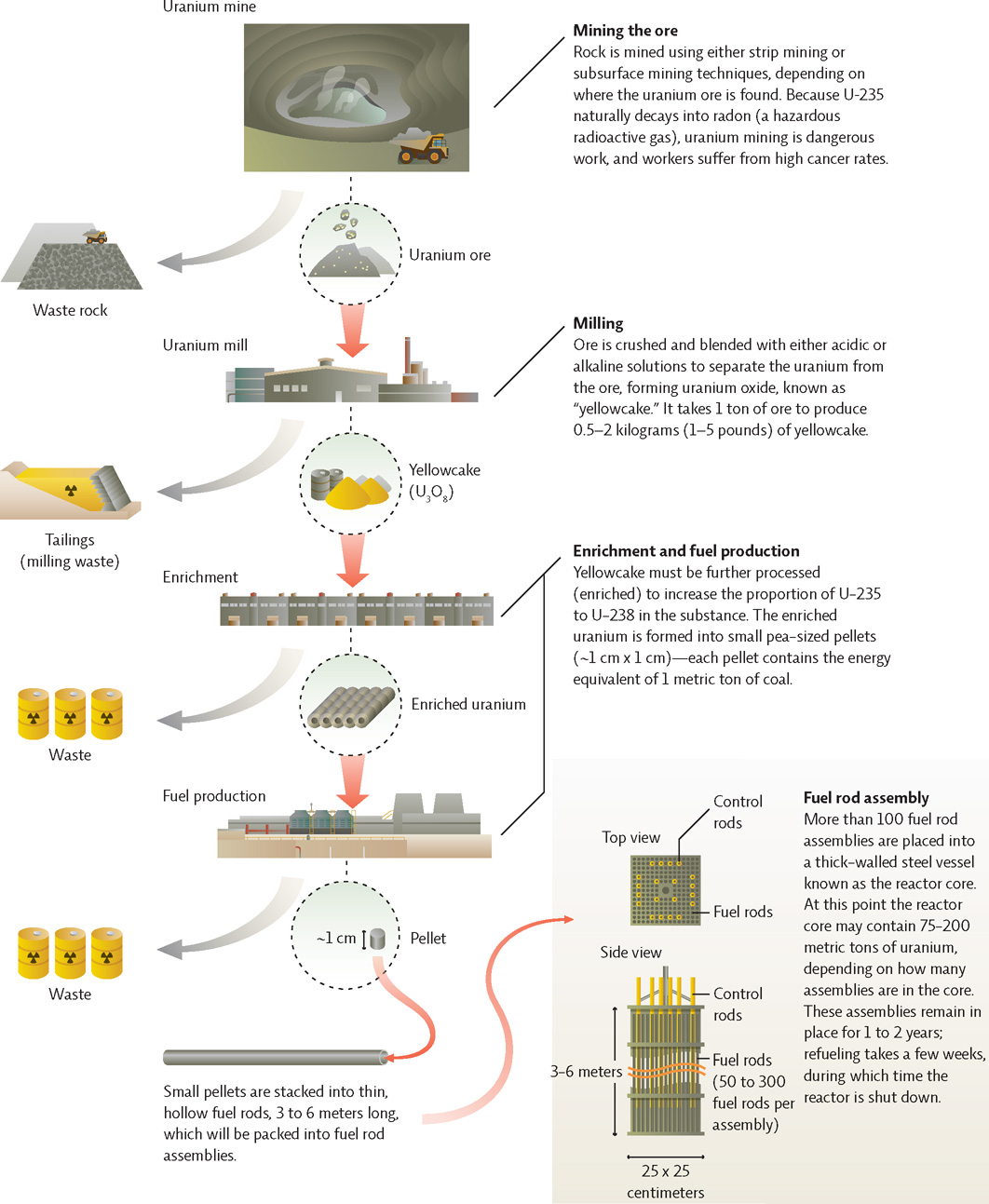

Which stages of nuclear fuel production have radioactive material present?
All of them (but it becomes more concentrated after the enrichment step).
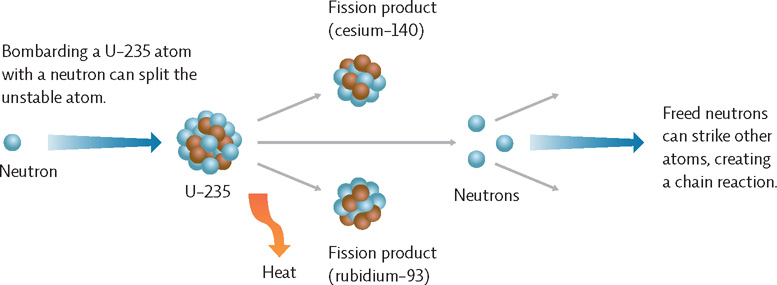
A nuclear fission chain reaction begins when U-235 in the fuel rods is deliberately bombarded with neutrons. This bombardment makes the uranium nucleus unstable, causing it to split into a variety of two or more smaller atoms and releasing two or three additional neutrons in the process. These newly released neutrons then hit other U-235 atoms, causing them to split and release even more neutrons, and so on.
Unlike the type of nuclear reaction at work in a nuclear bomb, which uses much more radioactive material, setting off a massive chain reaction that is almost instantaneous, the reactions at nuclear power plants are highly controlled. Control rods—made of materials such as boron or graphite that absorb neutrons—are placed in the fuel rod assembly between the fuel rods to control the speed of the reaction. They can be added (to slow down or stop the reaction) or removed (to make it go faster). INFOGRAPHIC 22.4
control rods
Rods that absorb neutrons and slow the fission chain reaction.
Fission, or the breaking apart of atoms, begins when an atom like U-235 is bombarded with a neutron. This breaks the atom into other smaller atoms and releases free neutrons, which in turn hit other U-235 atoms, causing them to split and release neutrons, and so on. The reaction in the fuel assembly is controlled by the insertion of control rods of nonfissionable material which absorb some of the free neutrons.

How do control rods help control the chain reaction in a nuclear fuel assembly?
The control rods absorb some of the neutrons without undergoing fission so fewer are zipping around, striking other radioactive isotopes.
KEY CONCEPT 22.4
In a nuclear fission reaction, U-235 is bombarded with neutrons to split the atoms; this releases more neutrons, which leads to a self-perpetuating chain reaction.
Even controlled, this chain reaction releases a tremendous amount of heat—10 million times more than would be released by burning a comparable amount of coal or oil. The heat is used to boil water, which produces steam, which turns turbines that create electricity.
All types of thermoelectric power take a lot of water; that’s why power plants are sited near rivers and oceans. But at the moment, nuclear power requires the most—on average, around 2,500 liters per megawatt hour (MWh), compared with 1,900 liters per MWh for coal and 600 liters per MWh for natural gas. (A MWh is the production of 1 megawatt— 1 million watts—over an hour’s time.) That means a typical 1,000-MW nuclear reactor requires 2,500,000 liters of water per minute to flow through the cooling system. Some reactors use much more. For example, each of the two 845-MW reactors at the Calvert Cliffs nuclear power plant in Maryland requires 4,500,000 liters (that’s 1,200,000 gallons) of water per minute during operation. Though most of this water (more than 95%) is returned to the source (river or ocean), there are problems with the release of warmer-than-normal water back into the environment, as well as damage to aquatic life that gets trapped in or against intake filters.
The reason for all that water is simple: With nuclear energy, water is needed not only to produce steam but also to keep spent fuel rods cool and to prevent the reactor from overheating. (Remember that heat is produced from radioactive decay of fission by-products, and it continues even after the reactor is shut down and fission stops; therefore, spent fuel needs constant cooling.) Without water to cool them, fuel rods can melt, releasing large amounts of radioactivity; the fuel rod metal casing can also get hot enough to react with steam in a way that produces highly explosive hydrogen gas.