
10.3 Carbohydrates Are Attached to Proteins to Form Glycoproteins
✓ 2 Differentiate among the types of glycoproteins in regard to structure and function.
A carbohydrate group can be covalently attached to a protein to form a glycoprotein. Such modifications are not rare, as 50% of the proteome consists of glycoproteins. We will examine three classes of glycoproteins. In the first class, referred to simply as glycoproteins, the protein constituent is the largest component by weight. This versatile class plays a variety of biochemical roles. Many glycoproteins are components of cell membranes, where they take part in processes such as cell adhesion and the binding of sperm to eggs. Other glycoproteins are formed by linking carbohydrates to soluble proteins. Many of the proteins secreted from cells are glycosylated, or modified by the attachment of carbohydrates, including most proteins present in the serum component of blood.
The second class of glycoproteins comprises the proteoglycans. The protein component of proteoglycans is conjugated to a particular type of polysaccharide called a glycosaminoglycan. Carbohydrates make up a much larger percentage by weight of the proteoglycan compared with simple glycoproteins. Proteoglycans function as structural components and lubricants.
The third class of glycoproteins are the mucins, or mucoproteins, which, like proteoglycans, are predominately carbohydrate. Mucins, a key component of mucus, serve as lubricants. N-Acetylgalactosamine is usually the carbohydrate moiety bound to the protein in mucins. N-Acetylgalactosamine is an example of an amino sugar, so named because an amino group replaces a hydroxyl group.
Carbohydrates May Be Linked to Asparagine, Serine, or Threonine Residues of Proteins
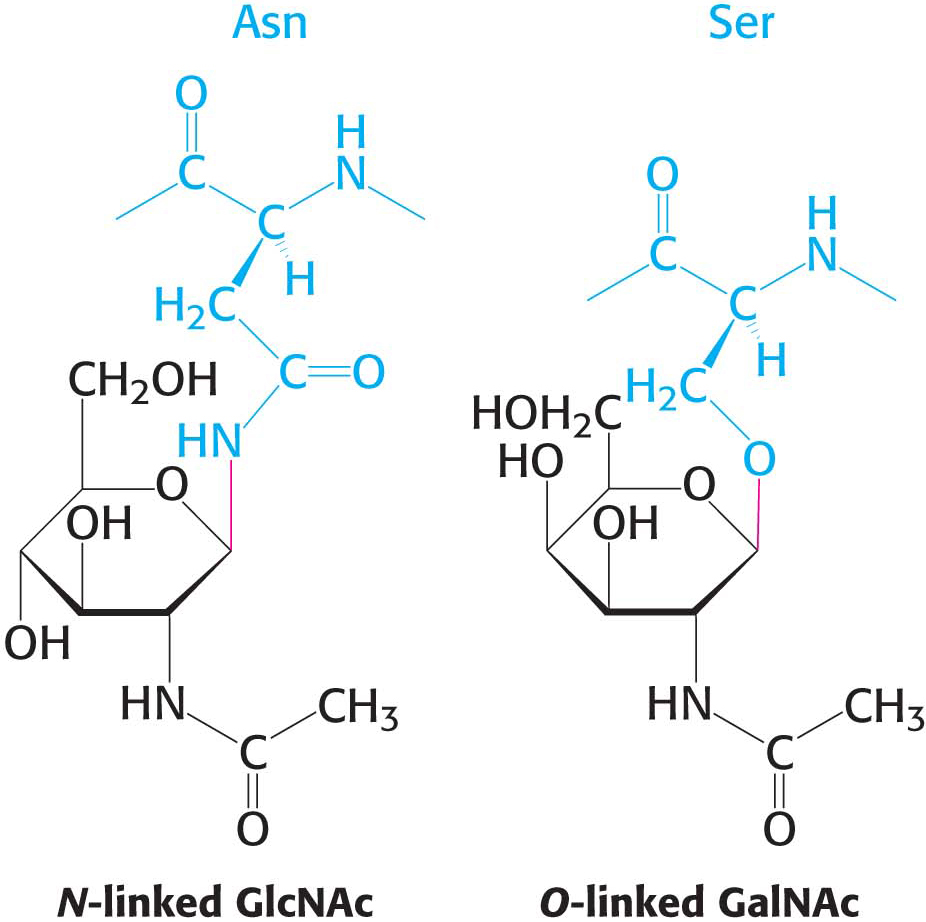
In all classes of glycoproteins, sugars are attached either to the amide nitrogen atom in the side chain of asparagine (termed an N-
 CLINICAL INSIGHT
CLINICAL INSIGHTThe Hormone Erythropoietin Is a Glycoprotein
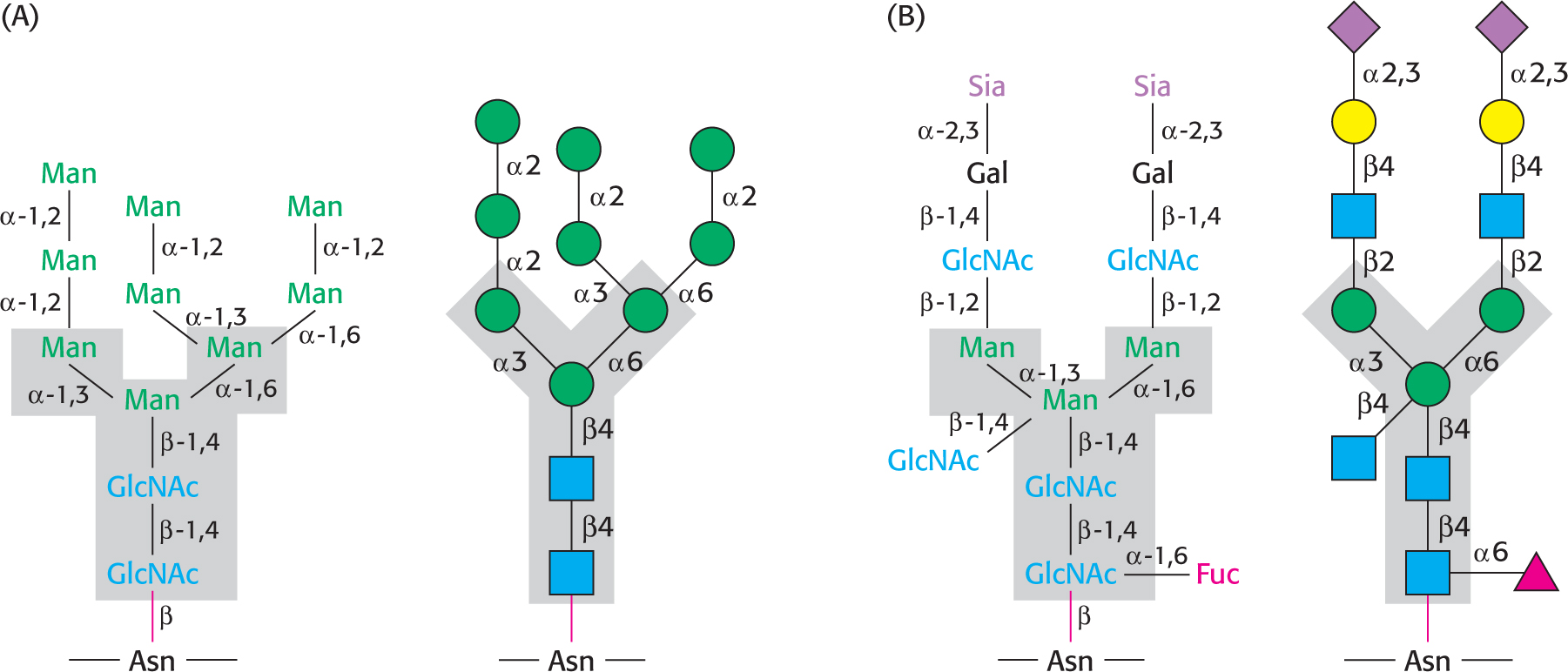
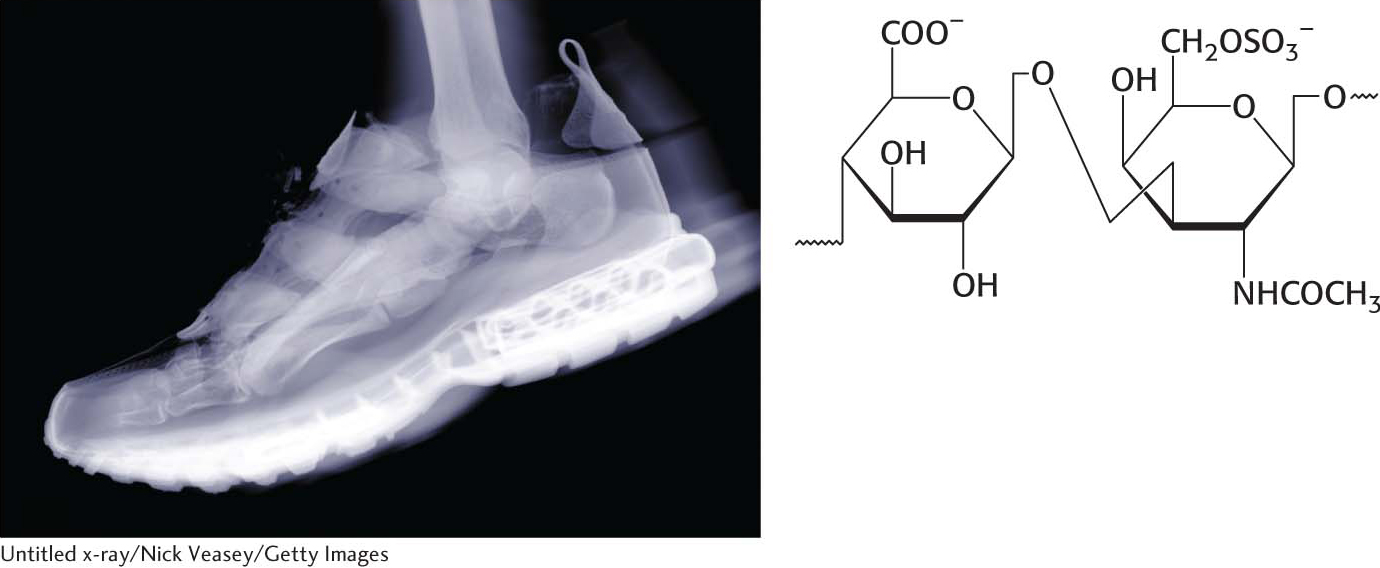
Let us look at a glycoprotein present in the blood serum that has dramatically improved treatment for anemia, particularly that induced by cancer chemotherapy. The glycoprotein hormone erythropoietin (EPO) is secreted by the kidneys and stimulates the production of red blood cells by bone marrow, the tissue in interior of bones. EPO is composed of 165 amino acids and is N-glycosylated at three asparagine residues and O-glycosylated on a serine residue (Figure 10.17), making the mature EPO 40% carbohydrate by weight. Glycosylation enhances the stability of the protein in the blood; unglycosylated protein has only about 10% of the bioactivity of the glycosylated form because the protein is rapidly removed from the blood by the kidney. The availability of recombinant human EPO has greatly aided the treatment of anemias. Some endurance athletes have used recombinant human EPO to increase the red-
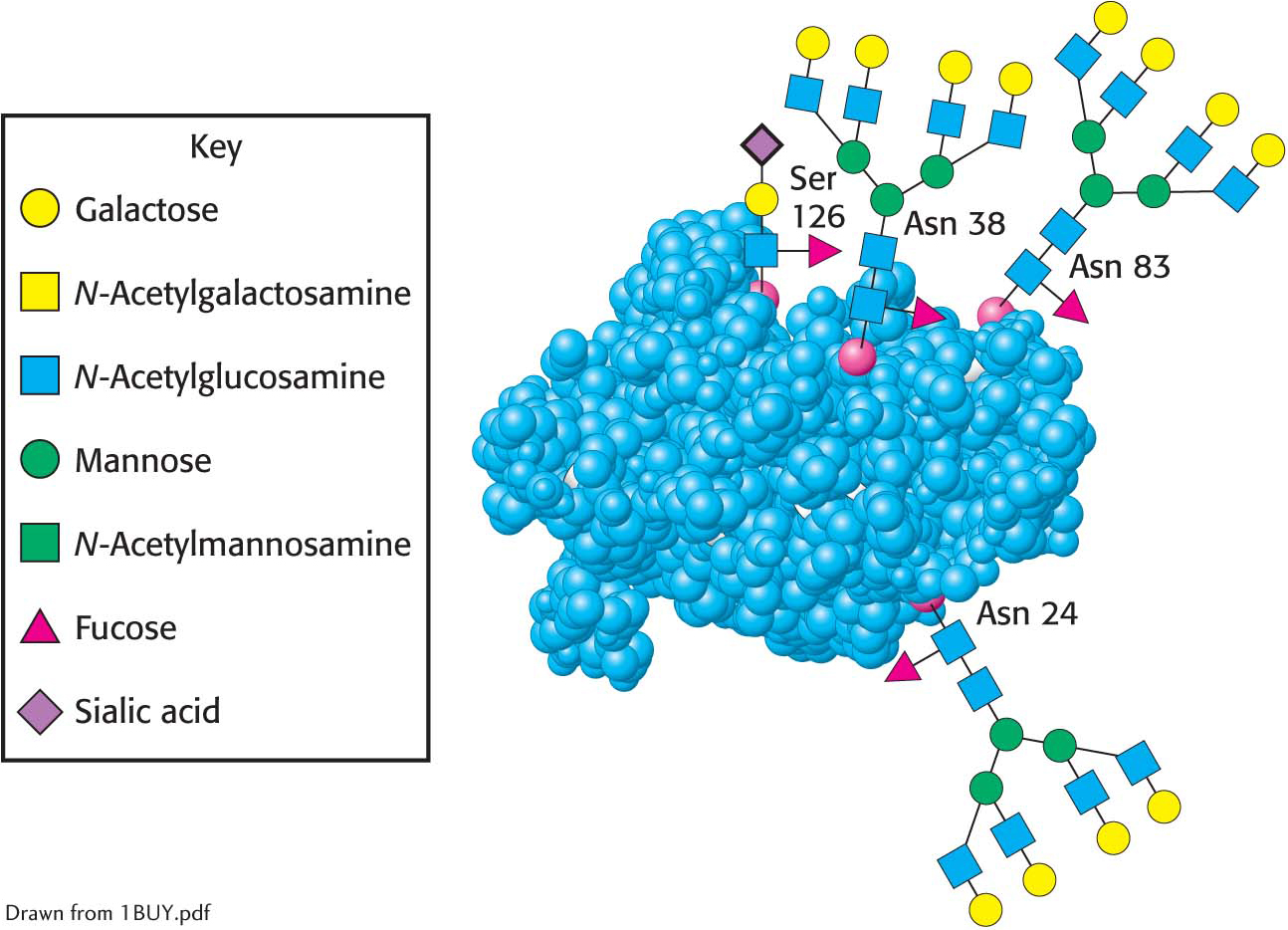
 Oligosaccharides attached to erythropoietin. Erythropoietin has oligosaccharides linked to three asparagine residues and one serine residue. The structures shown are approximately to scale. The carbohydrate structures represented in the amino acid residues are depicted symbolically by employing a scheme (shown in the key, which also applies to Figures 10.16, 10.23, and 10.24) that is becoming widely used.
Oligosaccharides attached to erythropoietin. Erythropoietin has oligosaccharides linked to three asparagine residues and one serine residue. The structures shown are approximately to scale. The carbohydrate structures represented in the amino acid residues are depicted symbolically by employing a scheme (shown in the key, which also applies to Figures 10.16, 10.23, and 10.24) that is becoming widely used.
Proteoglycans, Composed of Polysaccharides and Protein, Have Important Structural Roles
The second class of glycoproteins that we consider comprises the proteoglycans, proteins attached to a particular type of polysaccharide called glycosaminoglycans, as stated earlier. The glycosaminoglycan makes up as much as 95% of the biomolecule by weight, and so the proteoglycan resembles a polysaccharide more than a protein. Proteoglycans not only function as lubricants and structural components in connective tissue, but also mediate the adhesion of cells to the extracellular matrix, and bind factors that regulate cell proliferation.
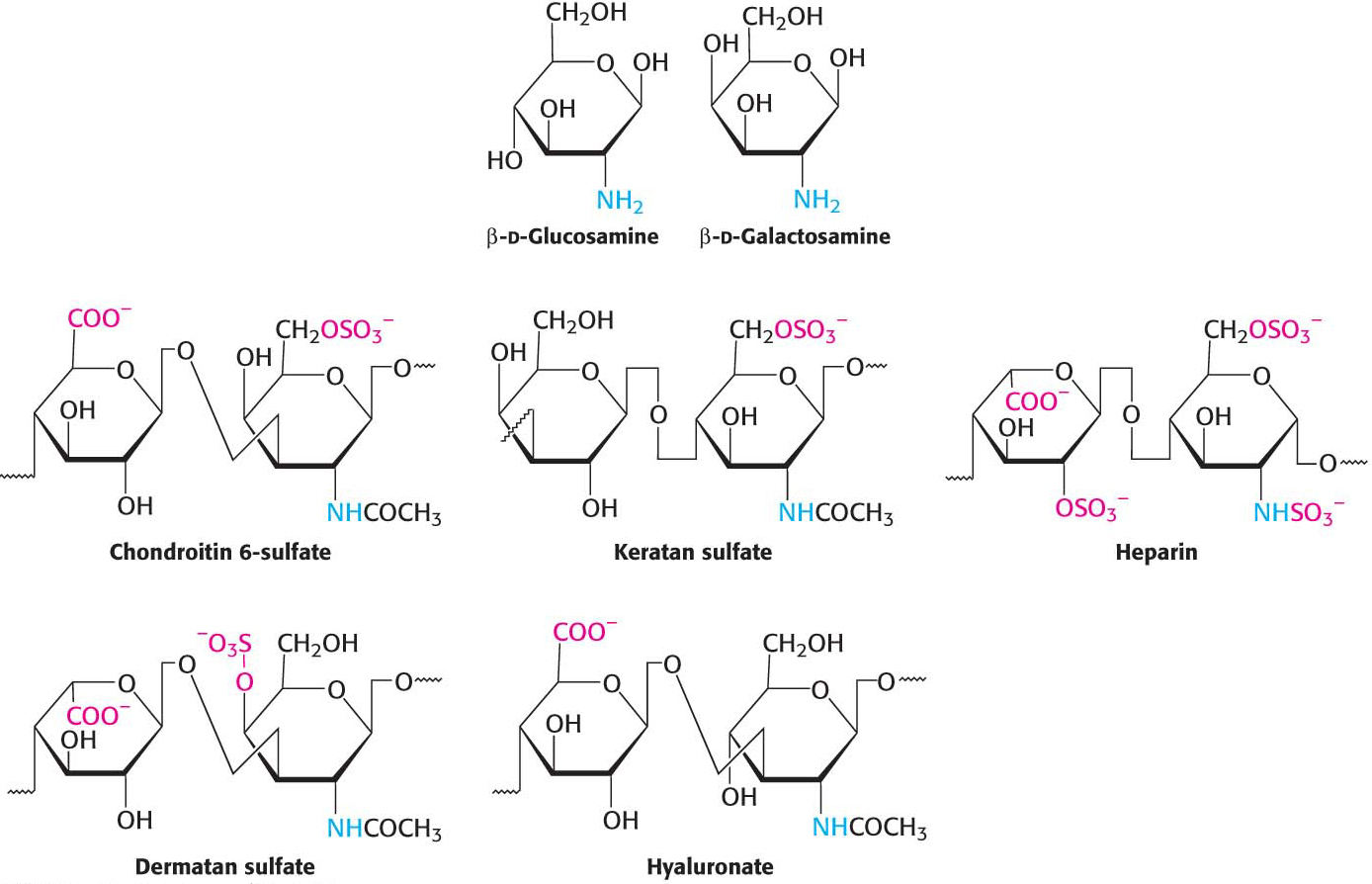
The properties of proteoglycans are determined primarily by the glycosaminoglycan component. Many glycosaminoglycans are made of repeating units of disaccharides containing a derivative of an amino sugar, either glucosamine or galactosamine (Figure 10.18). At least one of the two sugars in the repeating unit has a negatively charged carboxylate or sulfate group. The major glycosaminoglycans in animals are chondroitin sulfate, keratan sulfate, heparin, heparan sulfate, dermatan sulfate, and hyaluronate. Mucopolysaccharidoses are a collection of diseases, such as Hurler disease, that result from the inability to degrade glycosaminoglycans (Figure 10.19). Although precise clinical features vary with the disease, all mucopolysaccharidoses result in skeletal deformities and reduced life expectancies.

 CLINICAL INSIGHT
CLINICAL INSIGHTProteoglycans Are Important Components of Cartilage
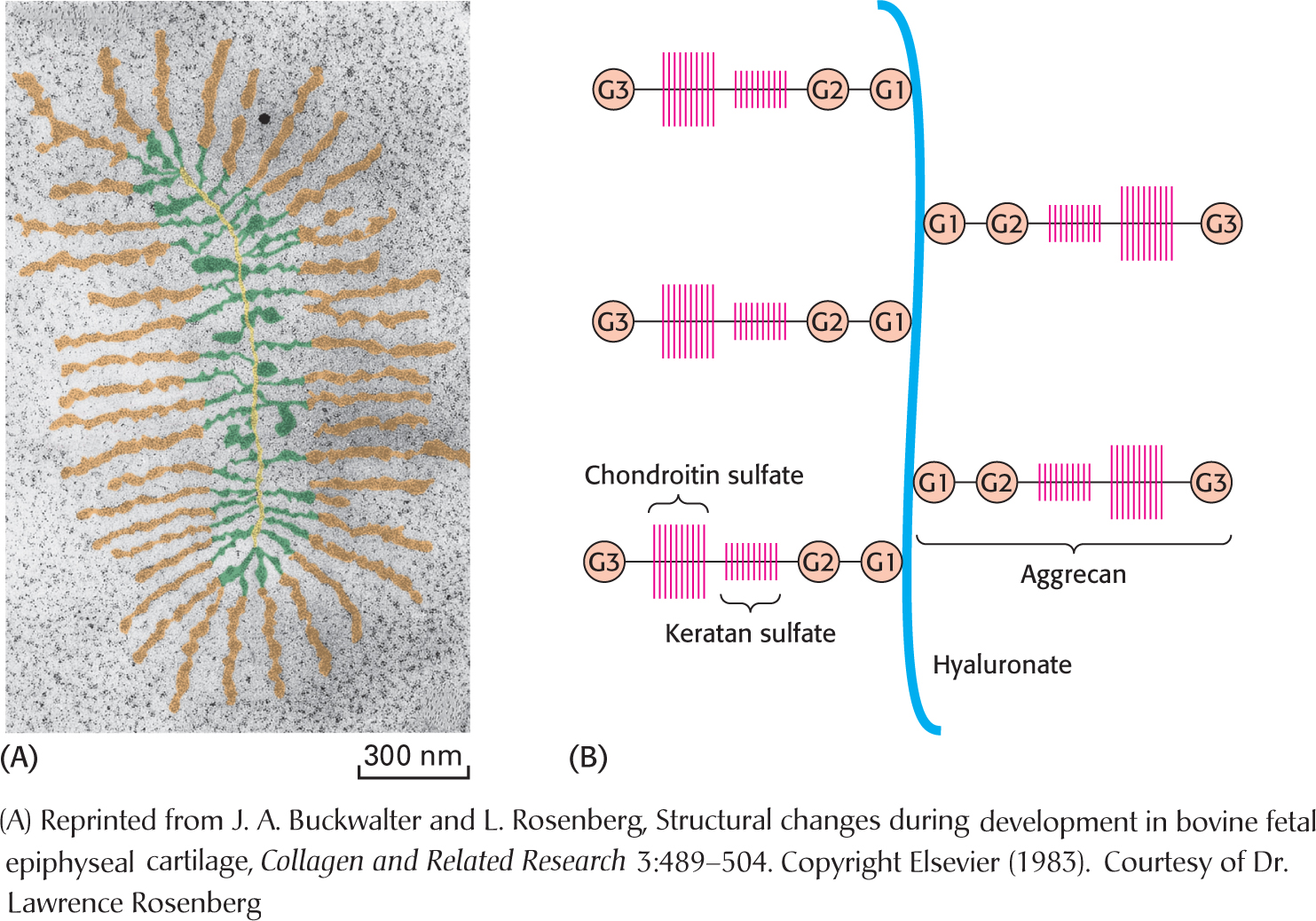
The proteoglycan aggrecan and the protein collagen are key components of cartilage. The triple helix of collagen provides structure and tensile strength, whereas aggrecan serves as a shock absorber (Figure 10.20). The protein component of aggrecan is a large molecule composed of 2397 amino acids (Figure 10.21). Many aggrecans are linked together by hyaluronate, a glycosaminoglycan. The aggrecan molecule itself is decorated with the glycosaminoglycans chondroitin sulfate and keratan sulfate. This combination of glycosaminoglycan and protein is especially suited to function as a shock absorber. Water is absorbed on the glycosaminoglycans, attracted by the many negative charges. Aggrecan can cushion compressive forces because the absorbed water enables the proteoglycan to spring back after having been deformed. When pressure is exerted, such as when the foot hits the ground in walking, water is squeezed from the glycosaminoglycan, cushioning the impact. When the pressure is released, the water rebinds. Osteoarthritis, the most common type of arthritis, can result from the proteolytic degradation of aggrecan and collagen in the cartilage in the joints due to inflammation. More typically, the cause is simple “wear and tear.” Glucosamine and chondroitin (Figure 10.18) are widely promoted as a treatment for osteoarthritis, but the benefits to anyone but the producers and marketers of the supplement are very ambiguous.
Although glycosaminoglycans may not seem to be familiar molecules, they are common throughout the biosphere. Chitin is a glycosaminoglycan found in the exoskeleton of insects, crustaceans, and arachnids and is, next to cellulose, the second most abundant polysaccharide in nature (Figure 10.22). Cephalopods, such as squid, have razor sharp beaks, which are made of extensively cross-

 CLINICAL INSIGHT
CLINICAL INSIGHTMucins Are Glycoprotein Components of Mucus
The third class of glycoproteins that we examine comprises the mucins (mucoproteins). In mucins, the protein component is extensively glycosylated to serine or threonine residues by N-acetylgalactosamine (Figure 10.7). Mucins are capable of forming large polymeric structures and are common in mucous secretions. These glycoproteins are synthesized by specialized cells in the tracheobronchial, gastrointestinal, and urogenital tracts. Mucins are abundant in saliva where they function as lubricants.
A model of a mucin is shown in Figure 10.23A. The defining feature of the mucins is a region of the protein backbone termed the variable number of tandem repeats (VNTR) region, which is rich in serine and threonine residues that are O-glycosylated. Indeed, the carbohydrate moiety can account for as much as 80% of the molecule by weight. A number of core carbohydrate structures are conjugated to the protein component of mucin. Figure 10.23B shows one such structure.
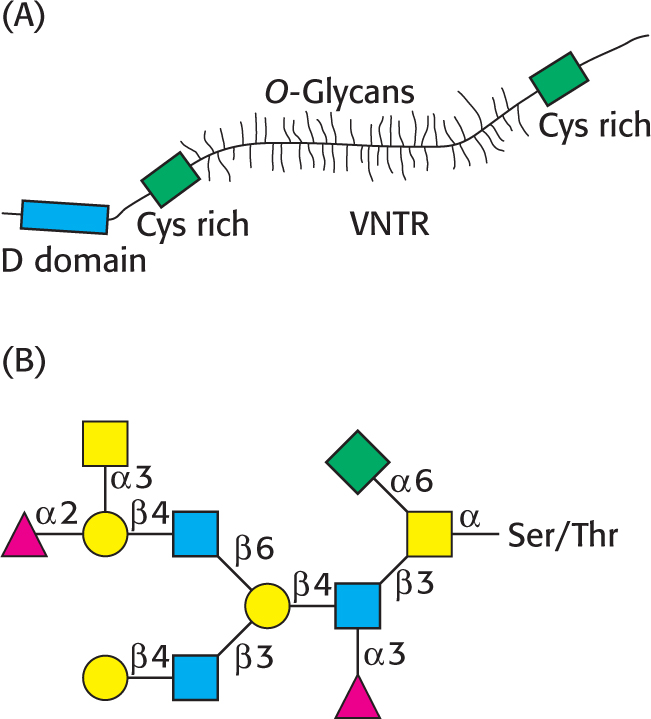
Mucins adhere to epithelial cells and act as a protective barrier; they also hydrate the underlying cells. In addition to protecting cells from environmental insults, such as stomach acid, inhaled chemicals in the lungs, and bacterial infections, mucins have roles in fertilization, the immune response, and cell adhesion. Mucins are overexpressed in bronchitis and cystic fibrosis, and the overexpression of mucins is also characteristic of adenocarcinomas—
QUICK QUIZ 2
Which amino acids are used for the attachment of carbohydrates to proteins?
Asparagine, serine, and threonine.
 BIOLOGICAL INSIGHT
BIOLOGICAL INSIGHTBlood Groups Are Based on Protein Glycosylation Patterns
The human ABO blood groups illustrate the effects of glycosyltransferases. Each blood group is designated by the presence of one of the three different carbohydrates, termed A, B, or O, on the surfaces of red blood cells (Figure 10.24). These structures have in common an oligosaccharide foundation called the O (or sometimes H) antigen. The A and B antigens differ from the O antigen by the addition of one extra monosaccharide, either N-acetylgalactosamine (for A) or galactose (for B) through an α-1,3 linkage to a galactose moiety of the O antigen.
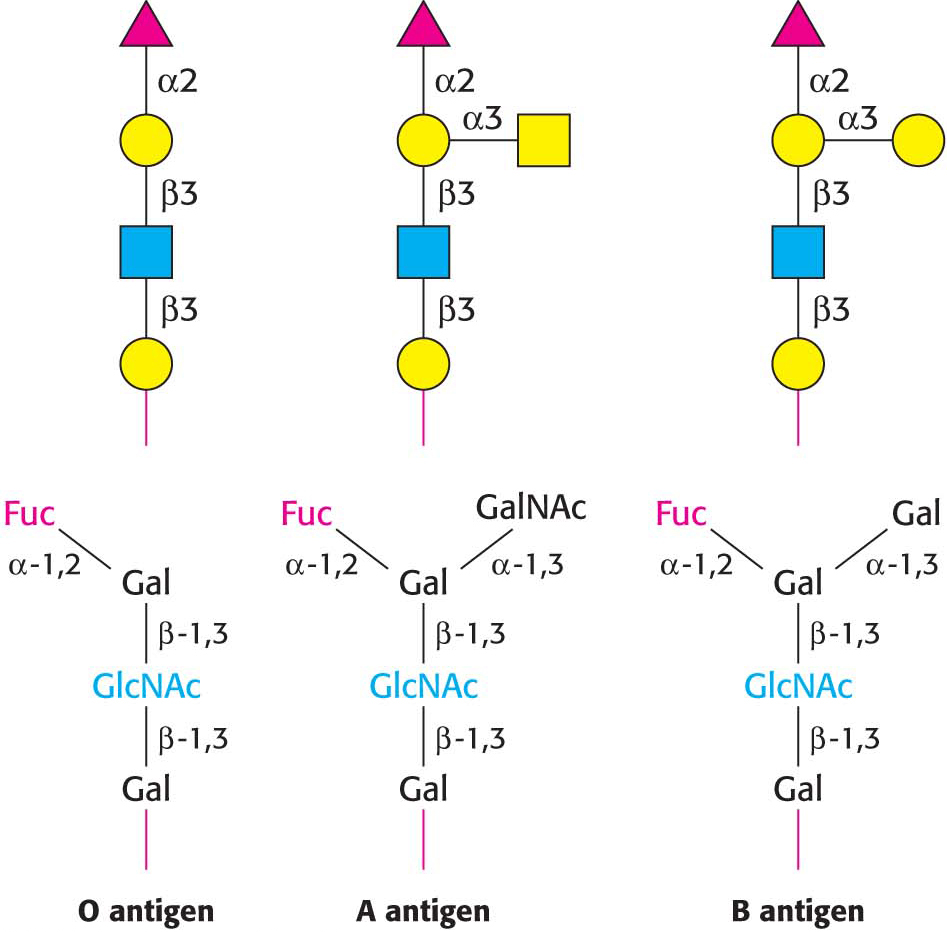
Specific glycosyltransferases add the extra monosaccharide to the O antigen. There are three common genes encoding such glycosyltransferases, and each person inherits the genes for each type from each parent. The type A transferase specifically adds N-acetylgalactosamine, whereas the type B transferase adds galactose. These enzymes are identical in all but 4 of 354 positions. The O phenotype is the result of a mutation in the O transferase that results in the synthesis of inactive enzyme.
These structures have important implications for blood transfusions and other transplantation procedures. If an antigen not normally present in a person is introduced, the person’s immune system recognizes it as foreign. Adverse reactions can ensue, initiated by the intravascular destruction of the incompatible red blood cells.
Why are different blood types present in the human population? Suppose that a pathogenic organism such as a parasite expresses on its cell surface a carbohydrate antigen similar to one of the blood-
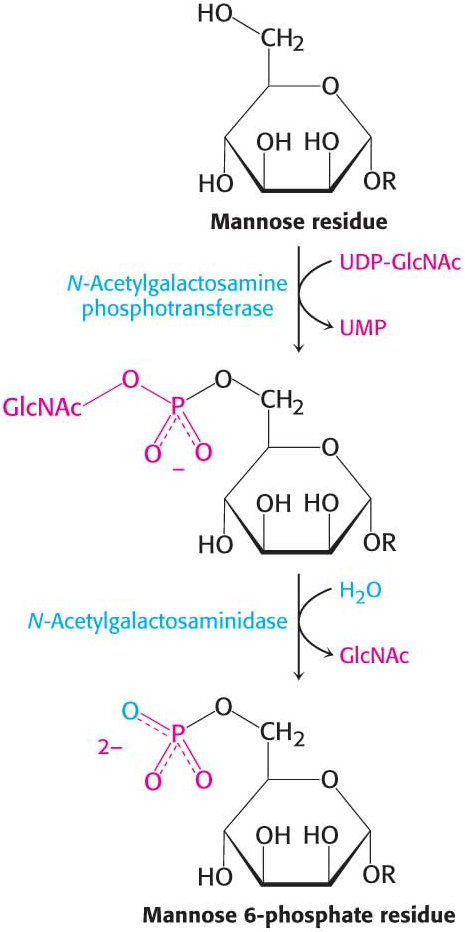
 CLINICAL INSIGHT
CLINICAL INSIGHTLack of Glycosylation Can Result in Pathological Conditions
Although the role of carbohydrate attachment to proteins is not known in detail in most cases, data indicate that this glycosylation is important for the processing and stability of these proteins, as it is for EPO. Certain types of muscular dystrophy can be traced to improper glycosylation of dystroglycan, a membrane protein that links the extracellular matrix with the cytoskeleton (Section 1.4). Indeed, an entire family of severe inherited human diseases called congenital disorders of glycosylation has been identified. These pathological conditions reveal the importance of proper modification of proteins by carbohydrates and their derivatives.
An especially clear example of the role of glycosylation is provided by I-