
18.2 The Pyruvate Dehydrogenase Complex Is Regulated by Two Mechanisms
✓ 2 Identify the means by which the pyruvate dehydrogenase complex is regulated.
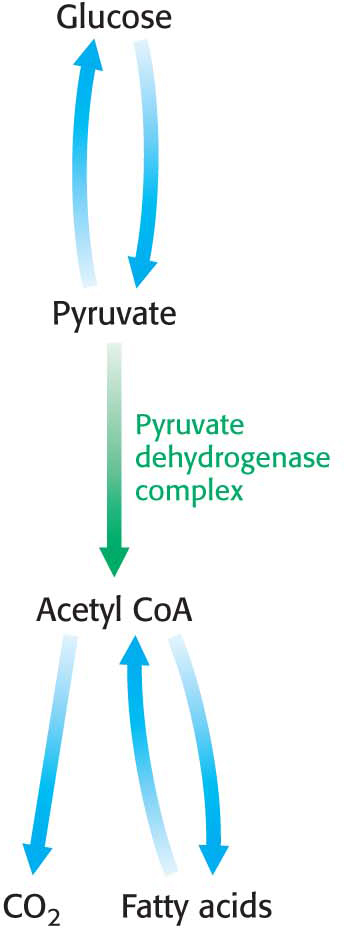
The pyruvate dehydrogenase complex is stringently regulated by multiple allosteric interactions and covalent modifications. As stated earlier, glucose can be formed from pyruvate through the gluconeogenic pathway. However, the formation of acetyl CoA from pyruvate is an irreversible step in animals and thus they are unable to convert acetyl CoA back into glucose. The oxidative decarboxylation of pyruvate to acetyl CoA commits the carbon atoms of glucose to either of two principal fates: (1) oxidation to CO2 by the citric acid cycle with the concomitant generation of energy or (2) incorporation into lipid, because acetyl CoA is a key precursor for lipid synthesis (Chapter 28 and Figure 18.8). High concentrations of reaction products inhibit the reaction: acetyl CoA inhibits the transacetylase component (E2) by directly binding to it, whereas NADH inhibits the dihydrolipoyl dehydrogenase (E3). High concentrations of NADH and acetyl CoA inform the enzyme that the energy needs of the cell have been met or that enough acetyl CoA and NADH have been produced from fatty acid degradation (Chapter 27). In either case, there is no need to metabolize pyruvate to acetyl CoA. This inhibition has the effect of sparing glucose, because most pyruvate is derived from glucose by glycolysis.
The key means of regulation of the complex in eukaryotes is covalent modification—
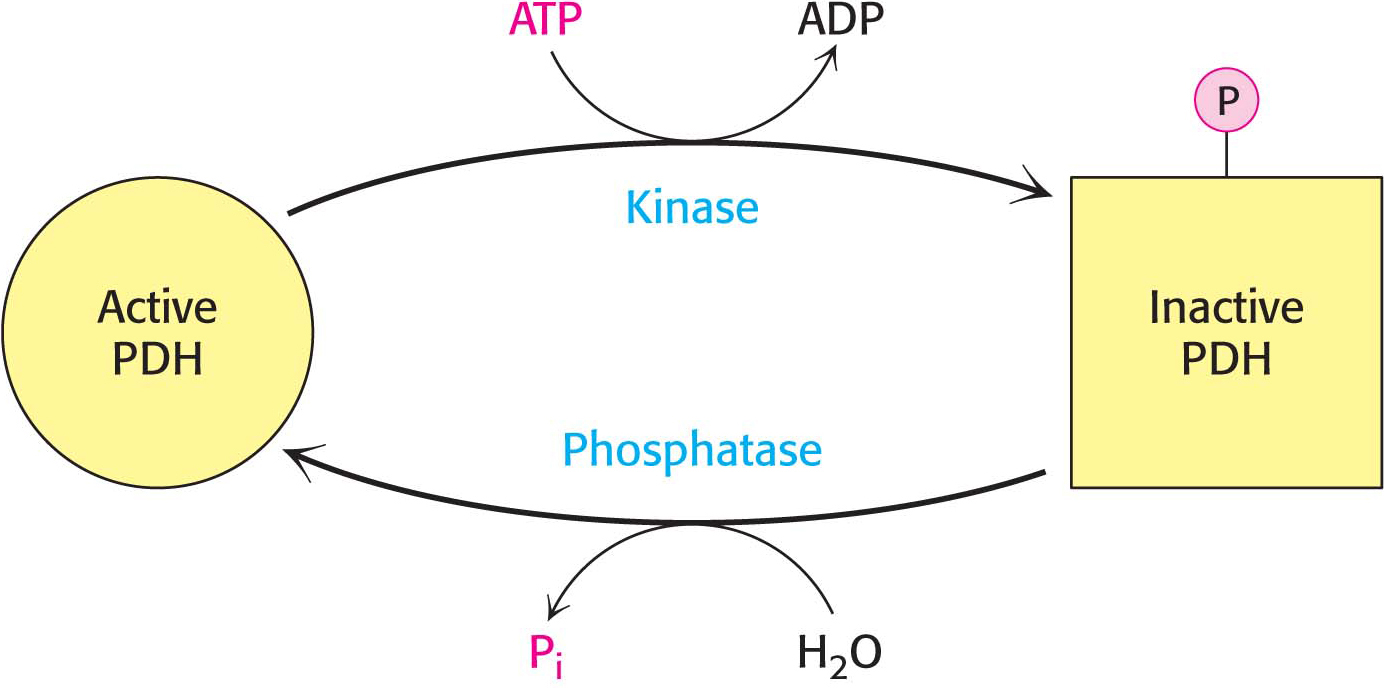
To see how this regulation works under biological conditions, consider muscle that is becoming active after a period of rest (Figure 18.10). At rest, the muscle will not have significant energy demands. Consequently, the NADH/NAD+, acetyl CoA/CoA, and ATP/ADP ratios will be high. These high ratios stimulate PDH kinase, promoting phosphorylation and, hence, deactivation of the pyruvate dehydrogenase complex. In other words, high concentrations of immediate (acetyl CoA and NADH) and ultimate (ATP) products of the pyruvate dehydrogenase complex inhibit its activity. Thus, pyruvate dehydrogenase is switched off when the energy charge is high.
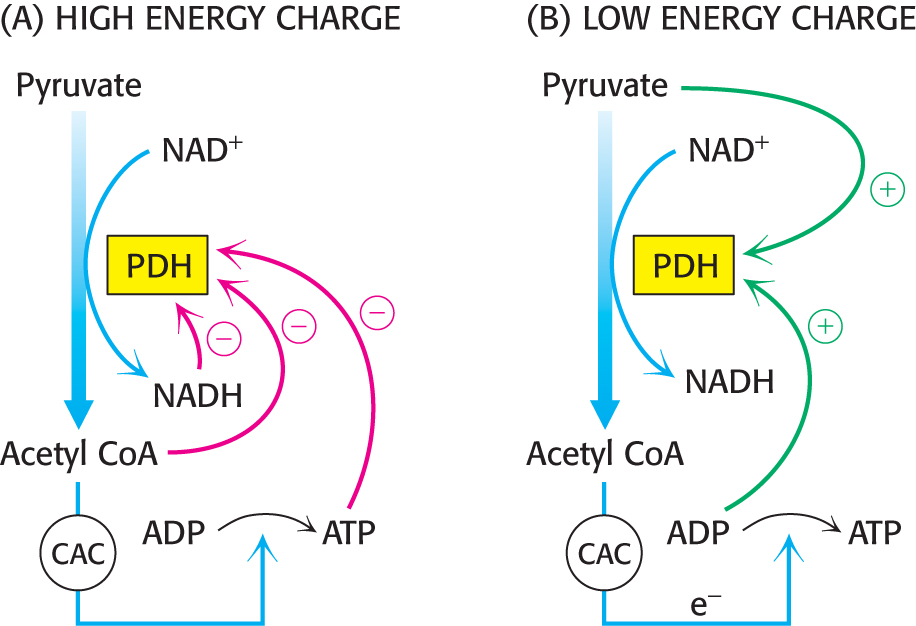
As exercise begins, the concentrations of ADP and pyruvate will increase as muscle contraction consumes ATP and glucose is converted into pyruvate to meet the energy demands. Both ADP and pyruvate activate the dehydrogenase by inhibiting PDH kinase. Moreover, the phosphatase is stimulated by Ca2+, a signal that also initiates muscle contraction. A rise in the cytoplasmic Ca2+ level to stimulate muscle contraction elevates the mitochondrial Ca2+ level. The rise in mitochondrial Ca2+ activates the phosphatase, enhancing pyruvate dehydrogenase activity.
In some tissues, the phosphatase is regulated by hormones. In liver, epinephrine binds to the α-adrenergic receptor to initiate the phosphatidylinositol pathway, causing an increase in Ca2+ concentration that activates the phosphatase. In tissues capable of fatty acid synthesis (such as the liver and adipose tissue), insulin (the hormone that signifies the fed state) stimulates the phosphatase, increasing the conversion of pyruvate into acetyl CoA. In these tissues, the pyruvate dehydrogenase complex is activated to funnel glucose to pyruvate and then to acetyl CoA and ultimately to fatty acids.
 CLINICAL INSIGHT
CLINICAL INSIGHTDefective Regulation of Pyruvate Dehydrogenase Results in Lactic Acidosis
In people with a phosphatase deficiency, pyruvate dehydrogenase is always phosphorylated and thus inactive. Consequently, glucose always has to take the anaerobic path to lactate rather than acetyl CoA. This condition results in unremitting lactic acidosis—high blood levels of lactic acid. In such an acidic environment, many tissues malfunction, most notably the central nervous system. One treatment for the condition is to place the patient on a ketogenic (high fat, adequate protein, low carbohydrate) diet to minimize the need to metabolize glucose.
QUICK QUIZ
List some of the advantages of organizing the enzymes that catalyze the formation of acetyl CoA from pyruvate into a single large complex.
The reaction is facilitated by having the active sites in proximity.
 CLINICAL INSIGHT
CLINICAL INSIGHTEnhanced Pyruvate Dehydrogenase Kinase Activity Facilitates the Development of Cancer
Recall that cancer cells metabolize glucose to lactate even in the presence of oxygen, a phenomenon called aerobic glycolysis or the Warburg effect. Under these conditions, the transcription factor hypoxia inducible factor-
DID YOU KNOW?
“A certain very troublesome affliction, which attacks men, is called by the inhabitants Beriberi (which means sheep). I believe those, whom this same disease attacks, with their knees shaking and the legs raised up, walk like sheep. It is a kind of paralysis, or rather Tremor: for it penetrates the motion and sensation of the hands and feet indeed sometimes of the whole body.”
—Jacob Bonitus, a physician working in Java in 1630

 CLINICAL INSIGHT
CLINICAL INSIGHTThe Disruption of Pyruvate Metabolism Is the Cause of Beriberi
The importance of the coordinated activity of the pyruvate dehydrogenase complex is illustrated by disorders that result from the absence of a key coenzyme. Recall that thiamine pyrophosphate is a coenzyme for the pyruvate dehydrogenase activity of the pyruvate dehydrogenase complex. Beriberi, a neurological and cardiovascular disorder, is caused by a dietary deficiency of thiamine (vitamin B1). Thiamine deficiency results in insufficient pyruvate dehydrogenase activity because thiamine pyrophosphate cannot be formed. The disease has been and continues to be a serious health problem in the Far East because rice, an important food there, has a rather low content of thiamine. This deficiency is partly ameliorated if the whole rice grain is soaked in water before milling; some of the thiamine in the husk then leaches into the rice kernel (Figure 18.11). The problem is exacerbated if the rice is polished, a practice that prevents spoilage and extends the storage life of rice, because only the outer layer contains significant amounts of thiamine. A form of beriberi, called Wernicke’s encephalopathy, is also occasionally seen in alcoholics who are severely malnourished and thus thiamine deficient. The disease is characterized by neurological and cardiac symptoms. Damage to the peripheral nervous system is expressed as pain in the limbs, weakness of the musculature, and distorted skin sensation. The heart may be enlarged and the cardiac output inadequate.
Thiamine pyrophosphate is not just crucial to the conversion of pyruvate to acetyl CoA. In fact, this coenzyme is the prosthetic group of three important enzymes: pyruvate dehydrogenase, a-ketoglutarate dehydrogenase (a citric acid cycle enzyme, Chapter 19), and transketolase. Transketolase functions in the pentose phosphate pathway, which will be considered in Chapter 26. The common feature of enzymatic reactions utilizing TPP is the transfer of an activated aldehyde unit. As expected in a body in which TPP is deficient, the levels of pyruvate and α-ketoglutarate in the blood of patients with beriberi are higher than normal. The increase in the level of pyruvate in the blood is especially pronounced after the ingestion of glucose. A related finding is that the activities of the pyruvate dehydrogenase complex and the α-ketoglutarate dehydrogenase complex in vivo are abnormally low. The low transketolase activity of red blood cells in beriberi is an easily measured and reliable diagnostic indicator of the disease.
Why does TPP deficiency lead primarily to neurological disorders? The nervous system relies essentially on glucose as its only fuel. The product of glycolysis—
Symptoms similar to those of beriberi appear in organisms exposed to mercury or arsenite (AsO33−). Both substances have a high affinity for sulfhydryls in close proximity to one another, such as those in the reduced dihydrolipoyl groups of the E3 component of the pyruvate dehydrogenase complex (Figure 18.12). The binding of mercury or arsenite to the dihydrolipoyl groups inhibits the complex and leads to central nervous system pathologies. The proverbial phrase “mad as a hatter” refers to the strange behavior of poisoned hat makers who used mercury nitrate to soften and shape animal furs (Figure 18.13). This form of mercury is absorbed through the skin. Similar symptoms afflicted the early photographers, who used vaporized mercury to create daguerreotypes.
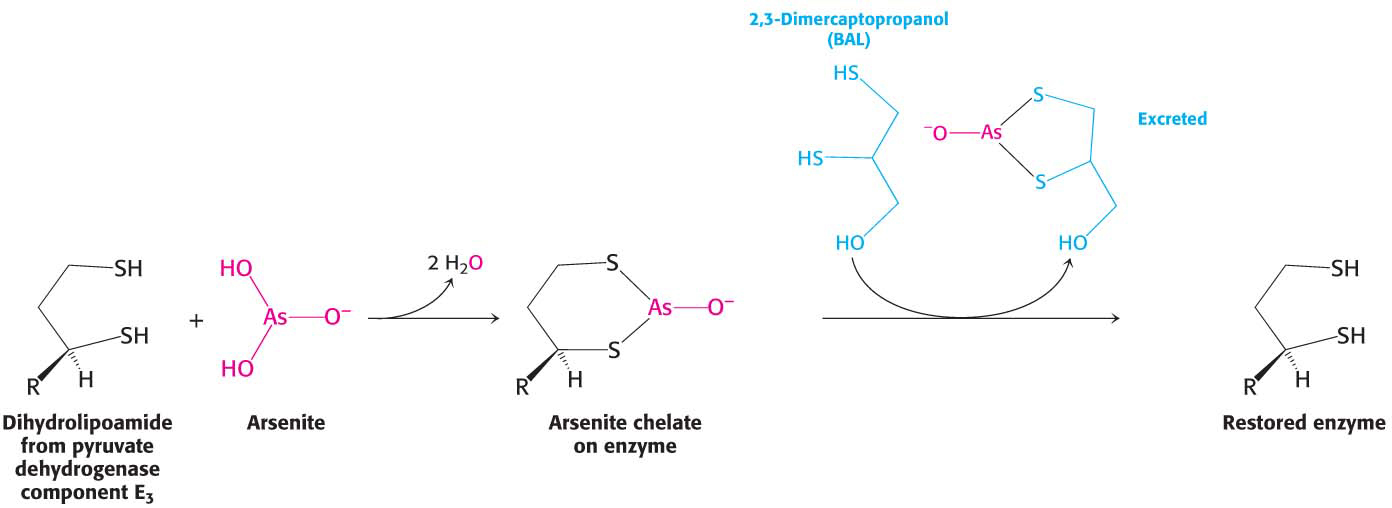

Treatment for these poisons is the administration of sulfhydryl reagents with adjacent sulfhydryl groups to compete with the dihydrolipoyl residues for binding with the metal ion. The reagent–