
4.4 Quaternary Structure: Multiple Polypeptide Chains Can Assemble into a Single Protein
Many proteins consist of more than one polypeptide chain in their functional states. Each polypeptide chain in such a protein is called a subunit. Quaternary structure refers to the arrangement of subunits and the nature of their interactions. The interactions among subunits of proteins displaying quaternary structure are usually the weak interactions discussed in Chapter 2: hydrogen bonds, ionic bonds, and van der Waals interactions.
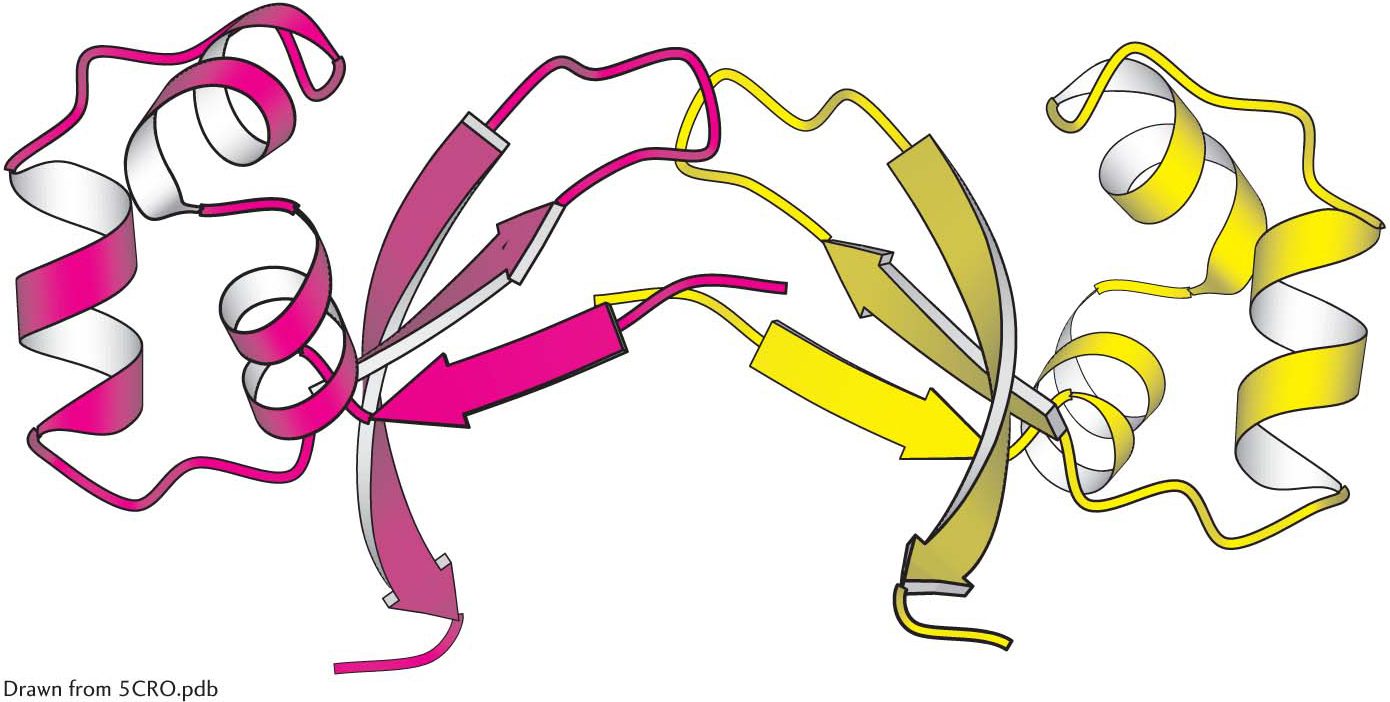
The simplest sort of quaternary structure is a dimer consisting of two identical subunits. This organization is present in Cro, a DNA-
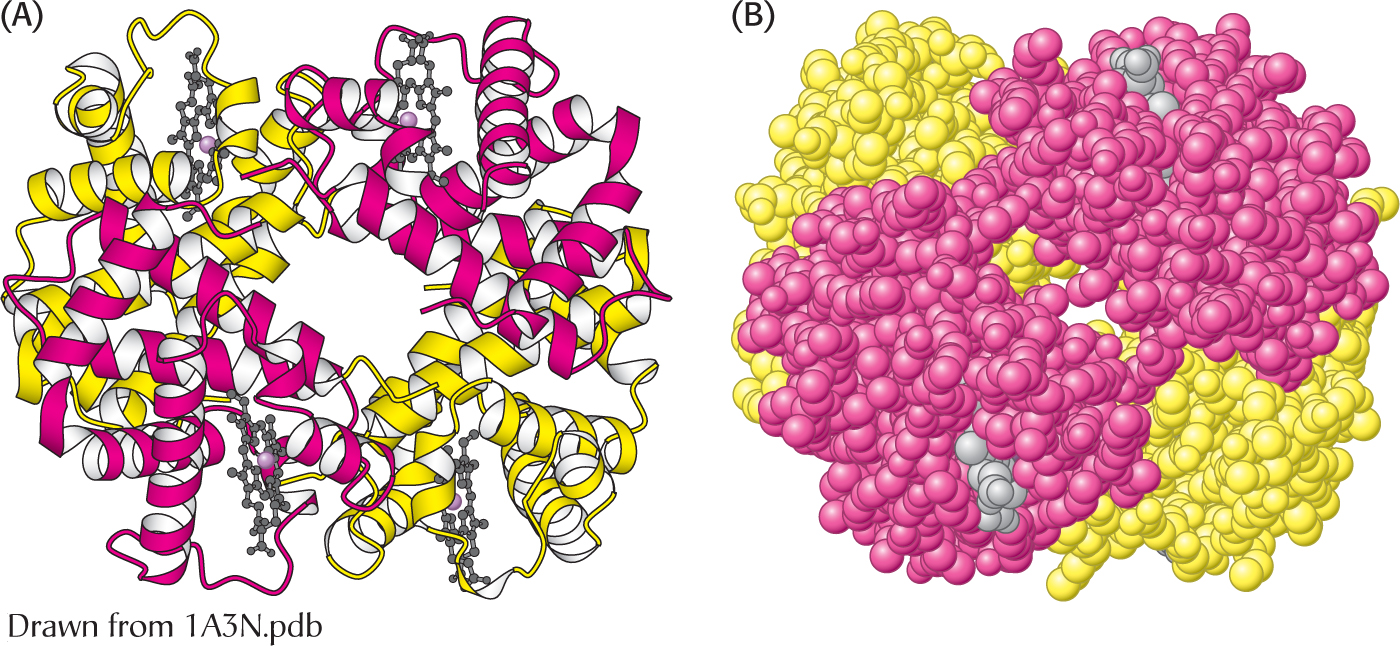

 The α2β2 tetramer of human hemoglobin. The structure of the two identical α subunits (red) and the two identical β subunits (yellow). (A) The ribbon diagram shows that they are composed mainly of α helices. (B) the space-
The α2β2 tetramer of human hemoglobin. The structure of the two identical α subunits (red) and the two identical β subunits (yellow). (A) The ribbon diagram shows that they are composed mainly of α helices. (B) the space-