9.5 Hydrogen Ions and Carbon Dioxide Promote the Release of Oxygen
2,3-
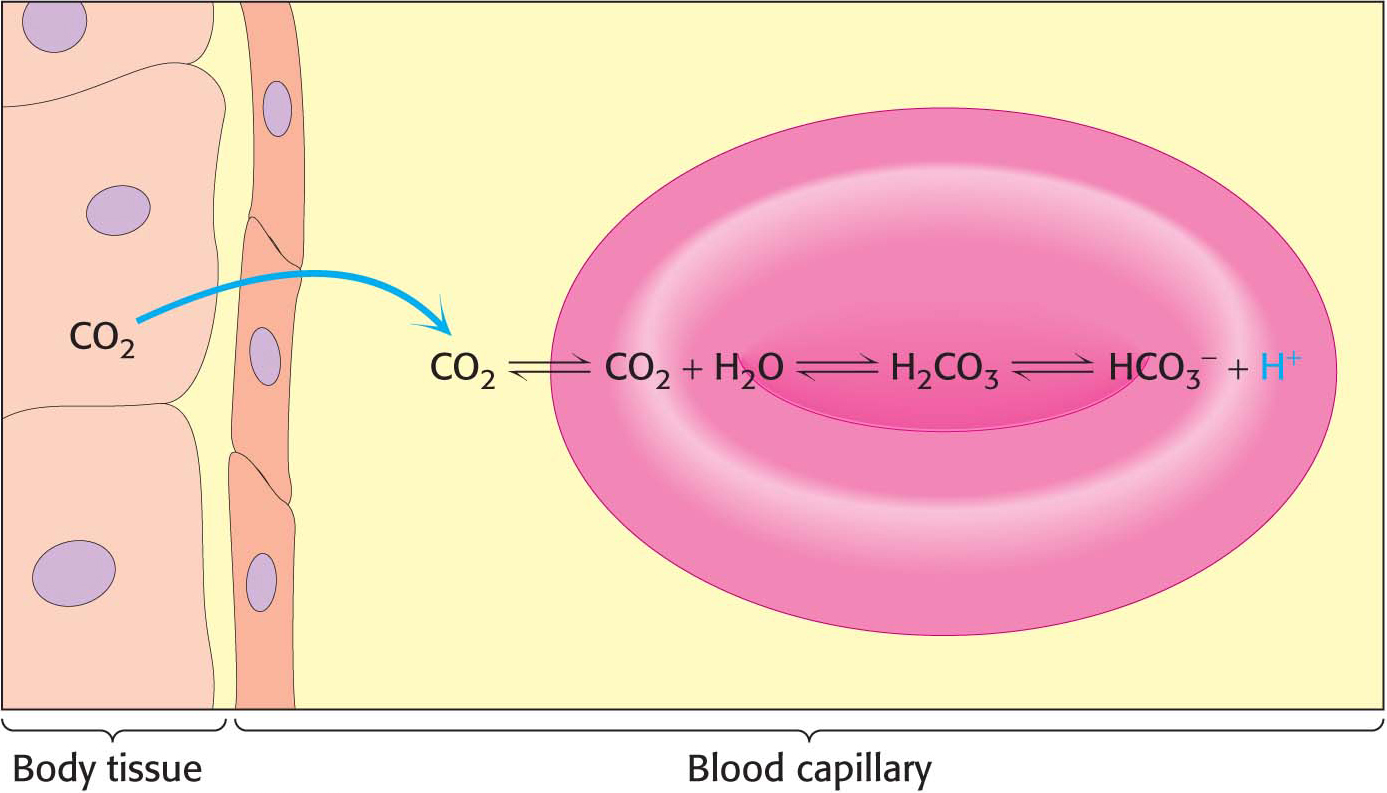
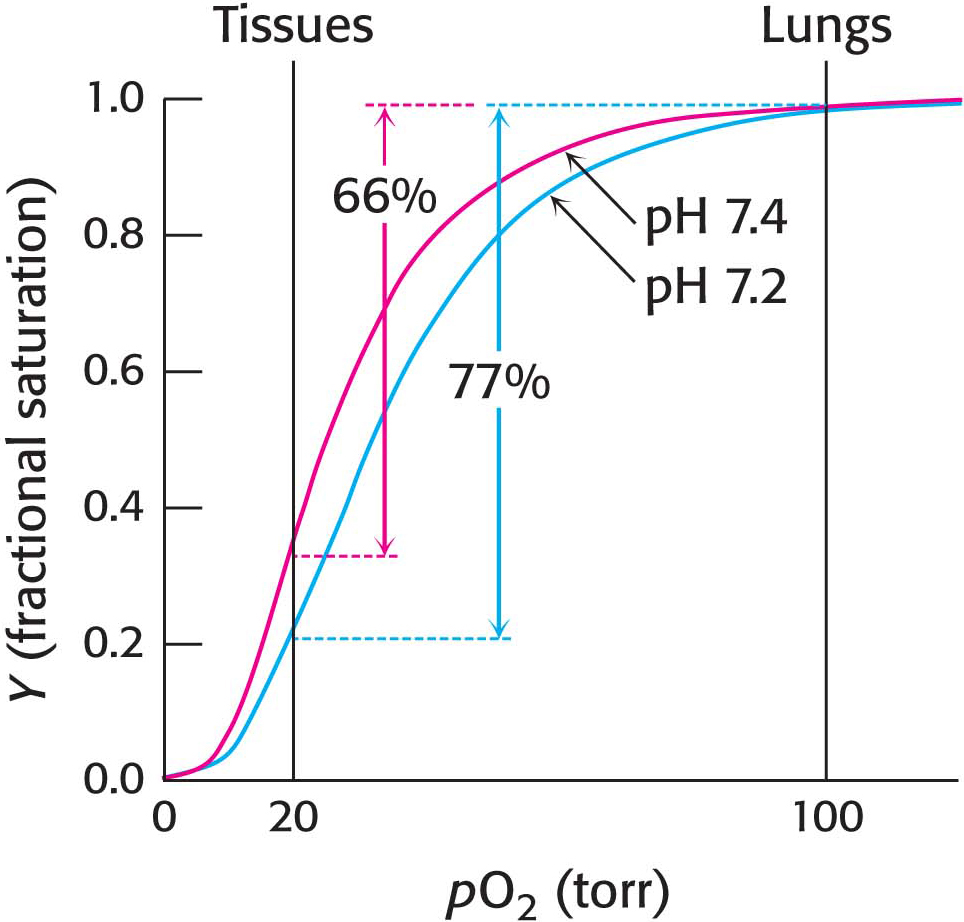
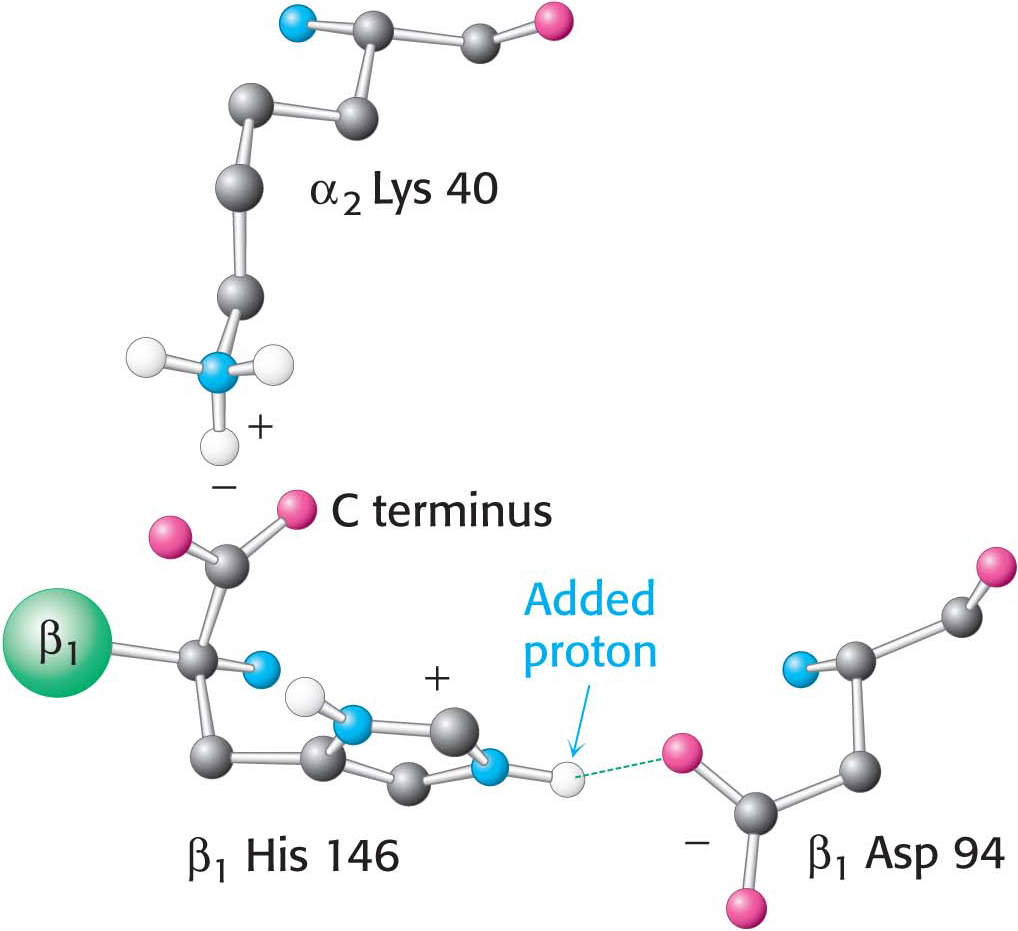
 Figure 9.15 The chemical basis of the Bohr effect. In deoxyhemoglobin, three amino acid residues form two salt bridges that stabilize the T quaternary structure. The formation of one of the salt bridges depends on the presence of an added proton on histidine β146. The proximity of the negative charge on aspartate β94 in deoxyhemoglobin favors the protonation of this histidine. Notice that the salt bridge between histidine β146 and aspartate β94 is stabilized by a hydrogen bond (green dashed line). The green sphere represents the remainder of the β1 chain.
Figure 9.15 The chemical basis of the Bohr effect. In deoxyhemoglobin, three amino acid residues form two salt bridges that stabilize the T quaternary structure. The formation of one of the salt bridges depends on the presence of an added proton on histidine β146. The proximity of the negative charge on aspartate β94 in deoxyhemoglobin favors the protonation of this histidine. Notice that the salt bridge between histidine β146 and aspartate β94 is stabilized by a hydrogen bond (green dashed line). The green sphere represents the remainder of the β1 chain.
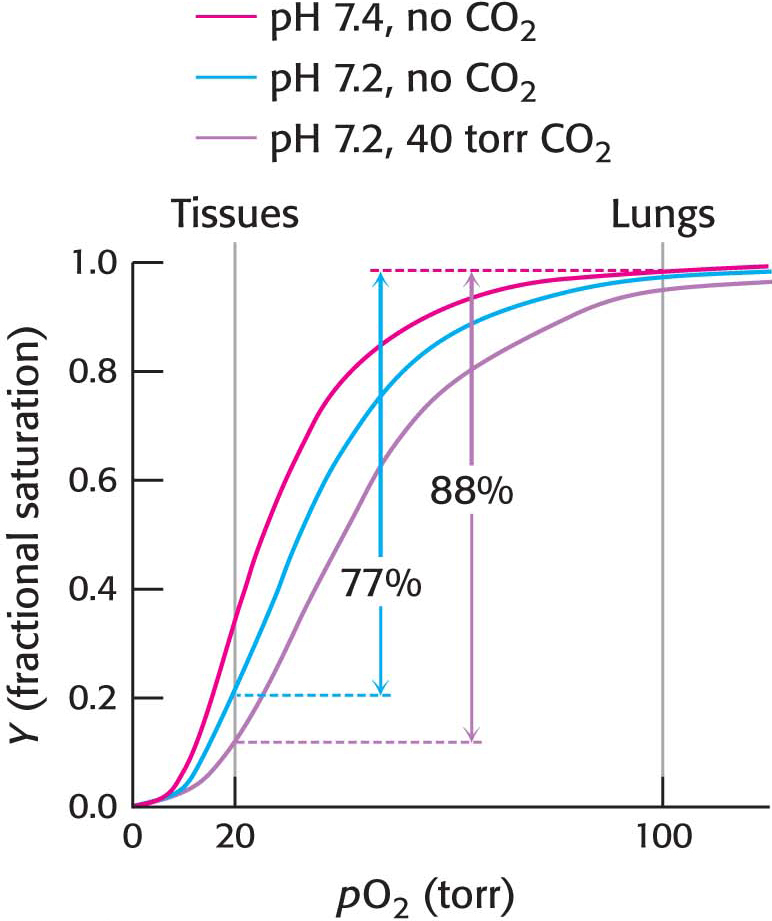
The oxygen affinity of hemoglobin decreases as pH decreases from the value of 7.4 found in the lungs, at 100 torr of oxygen partial pressure, to pH 7.2 and an oxygen partial pressure of 20 torr found at active muscle (Figure 9.14). This difference in pH and partial pressure results in a release of oxygen amounting to 77% of total carrying capacity. Recall that only 66% of the oxygen would be released in the absence of any change in pH. What are the chemical and structural bases of the pH effect? Deoxyhemoglobin is stabilized by ionic bonds, or salt bridges, two of which are shown in Figure 9.15. Consider the ionic bond between aspartate β94 and the protonated side chain of histidine β146. At high pH, the side chain of histidine β146 is not protonated and the salt bridge does not form, thus favoring oxygen binding. As the pH drops, as in the vicinity of actively metabolizing tissues, the side chain of histidine β146 becomes protonated, the salt bridge with aspartate β94 forms, and the quaternary structure characteristic of deoxyhemoglobin is stabilized, leading to a greater release of oxygen at actively metabolizing tissues. The ionic bond between the carboxyl terminus of histidine β146 and lysine α140 also stabilizes the T state. This bond is disrupted by changes elsewhere in the β1 chain that occur upon oxygen binding. Hemoglobin illustrates the fact that pH, as a cellular environmental factor, affects proteins other than enzymes.
In addition, hemoglobin responds to carbon dioxide with a decrease in oxygen affinity, thus facilitating the release of oxygen in tissues with a high carbon dioxide concentration, such as those in which fuel is actively undergoing combustion to form carbon dioxide and water. In the presence of carbon dioxide at a partial pressure of 40 torr, the amount of oxygen released approaches 90% of the maximum carrying capacity when the pH falls to 7.2 (Figure 9.16). Carbon dioxide stabilizes deoxyhemoglobin by reacting with the terminal amino groups to form carbamate groups, which are negatively charged.
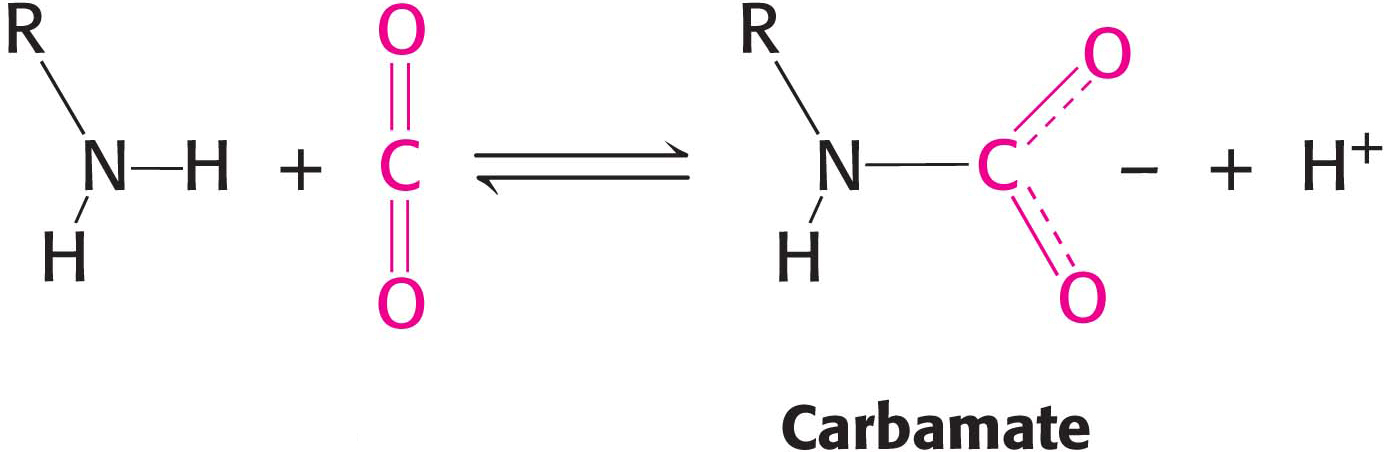
The amino termini lie at the interface between the αβ dimers. These negatively charged carbamate groups participate in salt-
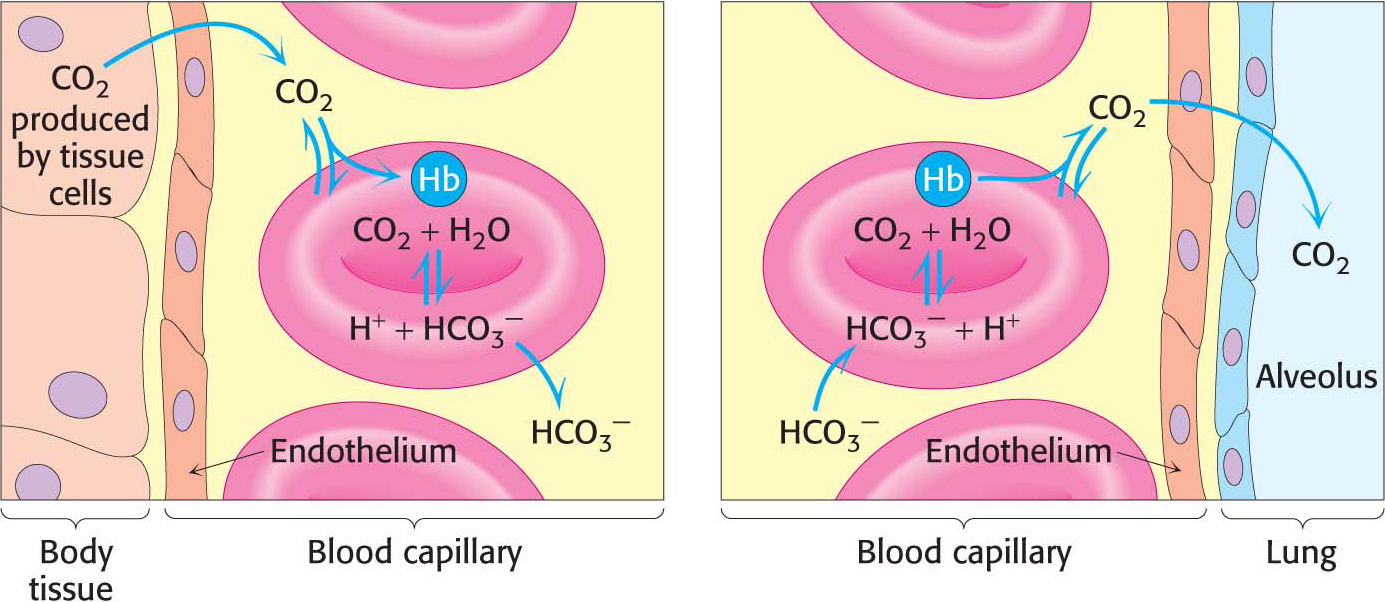
Hemoglobin with bound carbon dioxide and hydrogen ions is carried in the blood back to the lungs, where it releases the hydrogen ions and carbon dioxide and rebinds oxygen. Thus, hemoglobin helps to transport hydrogen ions and carbon dioxide in addition to transporting oxygen. However, transport by hemoglobin accounts for only about 14% of the total transport of these species; both hydrogen ions and carbon dioxide are also transported in the blood as bicarbonate (HCO3−) formed spontaneously or through the action of carbonic anhydrase, an enzyme abundant in red blood cells (Figure 9.17).
QUICK QUIZ
Name three factors that stabilize the deoxy form of hemoglobin.
BPG binding; salt bridges between acidic and basic amino acids; salt bridges that include amino-