26-5 A background of neutrinos and most of the helium in the universe are relics of the primordial fireball
The early universe must have been populated with vast numbers of neutrinos (ν) and their antiparticles, the antineutrinos (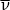 ). These particles have a very small mass, so their threshold temperature is quite low. These particles take part in the nuclear reactions that transform neutrons into protons and vice versa. For example, a neutron can decay into a proton by emitting an electron and an antineutrino:
). These particles have a very small mass, so their threshold temperature is quite low. These particles take part in the nuclear reactions that transform neutrons into protons and vice versa. For example, a neutron can decay into a proton by emitting an electron and an antineutrino:
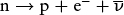
This radioactive decay happens quickly (its half-life is about 10.5 minutes), which is why we do not find free neutrons floating around in the universe today. In the first 2 seconds after the Big Bang, however, neutrons were also created by collisions between protons and electrons:
P + e− → n + ν
This reaction kept the number of neutrons approximately equal to the number of protons. This balance was maintained only as long as collisions between protons and electrons were frequent. But the number of electrons decreased precipitously as the temperature of the universe fell below 6 × 109 K and electrons and positrons annihilated each other without being replenished (Section 26-4). By the time the universe was about 2 seconds old, no new neutrons were being formed, the natural tendency for neutrons to decay into protons took over, and the number of neutrons began to decline.
Spawning the First Nuclei
Before many of the neutrons could decay into protons, they began to combine with protons to form nuclei. Nuclei of helium, the first element more massive than hydrogen, consist of either two protons and two neutrons (4He) or two protons and a single neutron (3He). It is exceedingly improbable that two protons and one or two neutrons should all simultaneously collide with one another to form a helium nucleus. Instead, helium nuclei are built in a series of steps. The first step is to have a single proton and a single neutron combine to form deuterium (2H), sometimes called “heavy hydrogen.” A photon (γ) is emitted in this process, so we write this reaction as
The first atomic nuclei formed within a quarter-hour after the Big Bang
p + n → 2H + γ
Forming deuterium, however, does not immediately lead to the formation of helium. The problem is that deuterium nuclei are easily destroyed, because a proton and a neutron do not stick together very well. Indeed, in the early universe, high-energy gamma rays easily broke deuterium nuclei back down into independent protons and neutrons. As a result, the synthesis of helium could not get beyond the first step. This block to the creation of helium is called the deuterium bottleneck.
When the universe was about 3 minutes old, the background radiation had cooled enough that its photons no longer had enough energy to break up the deuterium. By this time, most of the neutrons had decayed into protons, and protons outnumbered neutrons by about 6 to 1. Because deuterium nuclei could now survive, the remaining neutrons combined with protons and rapidly produced helium. (The Cosmic Connections: The Proton-Proton Chain figure for Chapter 16 depicts a similar sequence of reactions that take place in the core of the present-day Sun.)
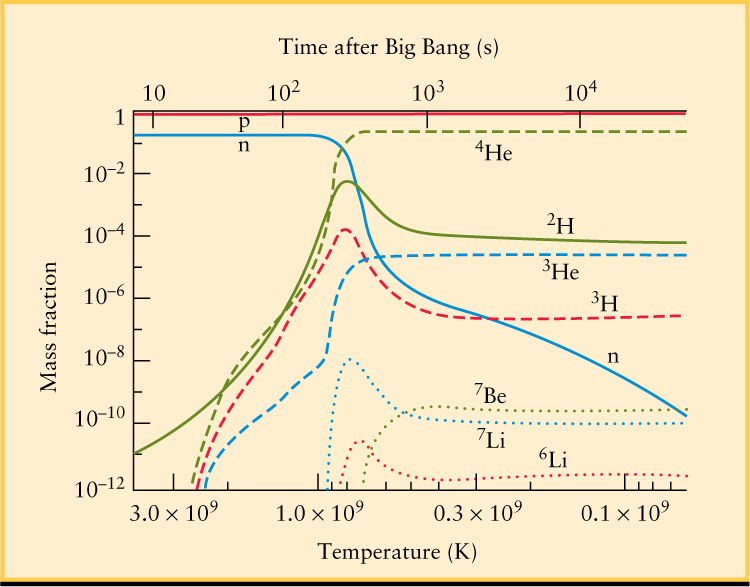
The result was what we find in the universe today—about 1 helium atom for every 10 hydrogen atoms. In addition to helium, nuclei of lithium (Li, which has 3 protons) and beryllium (Be, which has 4) were also produced in small numbers. The process of building up nuclei such as deuterium and helium from protons and neutrons is called nucleosynthesis (Figure 26-15).
Because nuclei have positive electric charges, bringing them together to form more massive nuclei requires that they overcome their mutual electric repulsion. They are unable to do so if they are moving too slowly, which will be the case if the temperature is too low. As a result, by about 15 minutes after the Big Bang the universe was no longer hot enough for nucleosynthesis to take place. Only the four lightest elements (hydrogen, helium, lithium, and beryllium) were present in appreciable numbers. The heavier elements would be formed only much later, once stars had formed and nuclear reactions within those stars could manufacture carbon, nitrogen, oxygen, and all the other elements.
Nucleosynthesis was one of the great early successes of the Big Bang theory. At lower temperatures (to the right in Figure 26-15), the amounts of different nuclei leveled off and remains unchanged. That allows us to compare the amount of each predicted atomic element to the amount observed today, and the agreement is excellent. Furthermore, these nucleosynthesis calculations also predicted the density of ordinary matter in the universe, and this prediction has been verified by the CMB fluctuations as in Figure 25-22 and Table 25-2. Taken together, the success of nucleosynthesis provides very strong evidence for a hot Big Bang, and that during its first few minutes the entire universe was one big nuclear reactor.
CAUTION!
Keep in mind that only nuclei formed in the first 15 minutes of the history of the universe. It would be another 380,000 years before temperatures became low enough for these nuclei to combine with electrons to form neutral atoms.
The Neutrino-Antineutrino Background
While nuclei were being formed in the early universe, what happened to all those primordial neutrinos and antineutrinos that had interacted so vigorously with the protons and neutrons before the universe was 2 seconds old? The answer is that by t = 2 seconds, matter was sufficiently spread out so that the universe became transparent to neutrinos and antineutrinos. From that time on, neutrinos and antineutrinos could travel across the universe unimpeded. Their interaction with matter is so weak that about a trillion neutrinos pass right through our bodies every second. Even Earth itself is virtually transparent to neutrinos from the Sun (Section 16-4).
The neutrinos and antineutrinos that were liberated at t = 2 seconds should now fill the universe much as the cosmic microwave background does. Indeed, these ancient neutrinos and antineutrinos may be about as populous today as the photons in the microwave background (of which there are 4.1 × 108 per cubic meter). The neutrino-antineutrino background should be slightly cooler than the photon background, which received extra energy from electron-positron annihilations. Physicists estimate that the current temperature of the neutrino-antineutrino background is about 2 K, as opposed to 2.725 K for the microwave background. Unfortunately, because neutrinos and antineutrinos are so difficult to detect, we do not yet have direct evidence of the neutrino-antineutrino background.
CONCEPT CHECK 26-9
How does the changing temperature of the universe confine the production of the elements helium, lithium, and beryllium to a period ranging from about 3 minutes to 15 minutes after the Big Bang?
The temperature of the universe steadily decreases with time as the universe expands. When the universe was less than 3 minutes old, hot radiation filling the universe was energetic enough to break apart protons and neutrons before they could begin to build atomic nuclei. However, the temperature also determines the average speed at which atomic nuclei collide into each other. After 15 minutes, the temperature was not high enough for colliding nuclei, with their charged protons, to overcome their electric repulsion and build larger nuclei.