Hemoglobin: Portrait of a Protein in Action
CHAPTER
7
191
The transition from anaerobic to aerobic life was a major step in evolution because it uncovered a rich reservoir of energy. Fifteen times as much energy is extracted from glucose in the presence of oxygen than in its absence. For single-
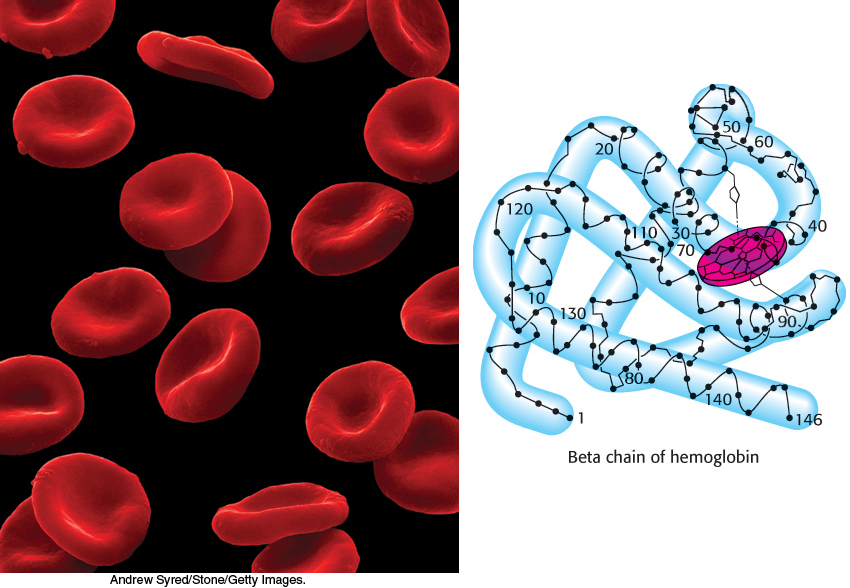
A comparison of myoglobin and hemoglobin illuminates some key aspects of protein structure and function. These two evolutionarily related proteins employ nearly identical structures for oxygen binding (Chapter 6). However, hemoglobin is a remarkably efficient oxygen carrier, able to use as much as 90% of its potential oxygen-
192
Hemoglobin and myoglobin have played important roles in the history of biochemistry. They were the first proteins for which three-