10.3Covalent Modification Is a Means of Regulating Enzyme Activity
Covalent Modification Is a Means of Regulating Enzyme Activity
The covalent attachment of a molecule to an enzyme or protein can modify its activity. In these instances, a donor molecule provides the functional moiety being attached. Most modifications are reversible. Phosphorylation and dephosphorylation are common means of covalent modification. The attachment of acetyl groups to lysine residues by acetyltransferases and their removal by deacetylases are another example. Histones—
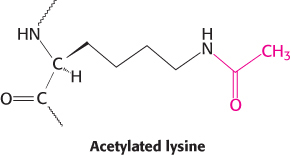
294
Modification is not readily reversible in some cases. The irreversible attachment of a lipid group causes some proteins in signal-
Virtually all the metabolic processes that we will examine are regulated in part by covalent modification. Indeed, the allosteric properties of many enzymes are altered by covalent modification. Table 10.1 lists some of the common covalent modifications.
|
Modification |
Donor molecule |
Example of modified protein |
Protein function |
|---|---|---|---|
|
Phosphorylation |
ATP |
Glycogen phosphorylase |
Glucose homeostasis; energy transduction |
|
Acetylation |
Acetyl CoA |
Histones |
DNA packing; transcription |
|
Myristoylation |
Myristoyl CoA |
Src |
Signal transduction |
|
ADP ribosylation |
NAD+ |
RNA polymerase |
Transcription |
|
Farnesylation |
Farnesyl pyrophosphate |
Ras |
Signal transduction |
|
γ-Carboxylation |
HCO_3 |
Thrombin |
Blood clotting |
|
Sulfation |
3′-Phosphoadenosine- |
Fibrinogen |
Blood- |
|
Ubiquitination |
Ubiquitin |
Cyclin |
Control of cell cycle |
Kinases and phosphatases control the extent of protein phosphorylation
We will see phosphorylation used as a regulatory mechanism in virtually every metabolic process in eukaryotic cells. Indeed, as much as 30% of eukaryotic proteins are phosphorylated. The enzymes catalyzing phosphorylation reactions are called protein kinases. These enzymes constitute one of the largest protein families known: there are more than 500 homologous kinases in human beings. This multiplicity of enzymes allows regulation to be fine-
ATP is the most common donor of phosphoryl groups. The terminal (γ) phosphoryl group of ATP is transferred to a specific amino acid of the acceptor protein or enzyme. In eukaryotes, the acceptor residue is commonly one of the three containing a hydroxyl group in its side chain. Transfers to serine and threonine residues are handled by one class of protein kinases and to tyrosine residues by another. Tyrosine kinases, which are unique to multicellular organisms, play pivotal roles in growth regulation, and mutations in these enzymes are commonly observed in cancer cells.
295
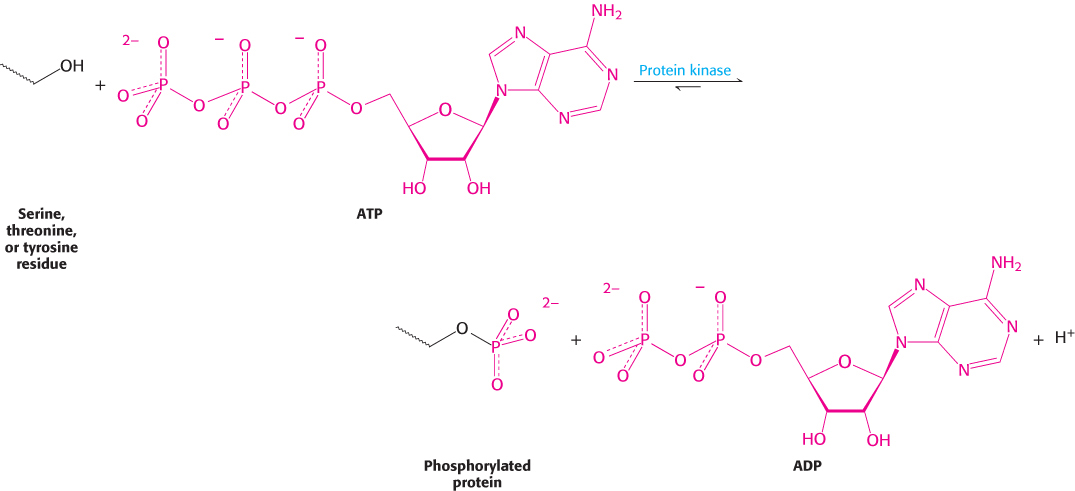
Table 10.2 lists a few of the known serine and threonine protein kinases. The acceptors in protein-
|
Signal |
Enzyme |
|---|---|
|
Cyclic nucleotides |
Cyclic AMP- Cyclic GMP- |
|
Ca2+ and calmodulin |
Ca2+−calmodulin protein kinase Phosphorylase kinase or glycogen synthase kinase 2 |
|
AMP |
AMP- |
|
Diacylglycerol |
Protein kinase C |
|
Metabolic intermediates and other “local” effectors |
Many target- |
|
Source: Information from D. Fell, Understanding the Control of Metabolism (Portland Press, 1997), Table 7.2. |
|
Protein kinases vary in their degree of specificity. Dedicated protein kinases phosphorylate a single protein or several closely related ones. Multifunctional protein kinases modify many different targets; they have a wide reach and can coordinate diverse processes. Comparisons of amino acid sequences of many phosphorylation sites show that a multifunctional kinase recognizes related sequences. For example, the consensus sequence recognized by protein kinase A is Arg-
Protein phosphatases reverse the effects of kinases by catalyzing the removal of phosphoryl groups attached to proteins. The enzyme hydrolyzes the bond attaching the phosphoryl group.
296
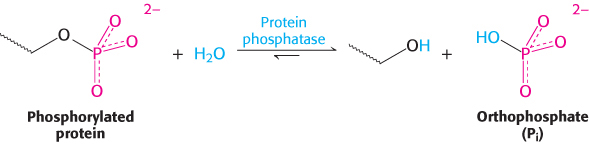
The unmodified hydroxyl-
Importantly, the phosphorylation and dephosphorylation reactions are not the reverse of one another; each is essentially irreversible under physiological conditions. Furthermore, both reactions take place at negligible rates in the absence of enzymes. Thus, phosphorylation of a protein substrate will take place only through the action of a specific protein kinase and at the expense of ATP cleavage, and dephosphorylation will take place only through the action of a phosphatase. The result is that target proteins cycle unidirectionally between unphosphorylated and phosphorylated forms. The rate of cycling between the phosphorylated and the dephosphorylated states depends on the relative activities of kinases and phosphatases.
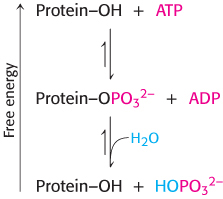
Phosphorylation is a highly effective means of regulating the activities of target proteins
Phosphorylation is a common covalent modification of proteins in all forms of life, which leads to the question, What makes protein phosphorylation so valuable in regulating protein function that its use is ubiquitous? Phosphorylation is a highly effective means of controlling the activity of proteins for several reasons:
The free energy of phosphorylation is large. Of the −50 kJ mol−1 (−12 kcal mol−1) provided by ATP, about half is consumed in making phosphorylation irreversible; the other half is conserved in the phosphorylated protein. A free-
energy change of 5.69 kJ mol−1 (1.36 kcal mol−1) corresponds to a factor of 10 in an equilibrium constant. Hence, phosphorylation can change the conformational equilibrium between different functional states by a large factor, of the order of 104. In essence, the energy expenditure allows for a stark shift from one conformation to another. A phosphoryl group adds two negative charges to a modified protein. These new charges may disrupt electrostatic interactions in the unmodified protein and allow new electrostatic interactions to be formed. Such structural changes can markedly alter substrate binding and catalytic activity.
A phosphoryl group can form three or more hydrogen bonds. The tetrahedral geometry of a phosphoryl group makes these bonds highly directional, allowing for specific interactions with hydrogen-
bond donors. Phosphorylation and dephosphorylation can take place in less than a second or over a span of hours. The kinetics can be adjusted to meet the timing needs of a physiological process.
Phosphorylation often evokes highly amplified effects. A single activated kinase can phosphorylate hundreds of target proteins in a short interval. If the target protein is an enzyme, it can in turn transform a large number of substrate molecules.
ATP is the cellular energy currency (Chapter 15). The use of this compound as a phosphoryl-
group donor links the energy status of the cell to the regulation of metabolism.
297
Cyclic AMP activates protein kinase A by altering the quaternary structure
Let us examine a specific protein kinase that helps animals cope with stressful situations. The “flight or fight” response is common to many animals presented with a dangerous or exciting situation. Muscle becomes primed for action. This priming is the result of the activity of a particular protein kinase. In this case, the hormone epinephrine (adrenaline) triggers the formation of cyclic AMP (cAMP), an intracellular messenger formed by the cyclization of ATP. Cyclic AMP subsequently activates a key enzyme: protein kinase A (PKA). The kinase alters the activities of target proteins by phosphorylating specific serine or threonine residues. The striking finding is that most effects of cAMP in eukaryotic cells are achieved through the activation of PKA by cAMP.
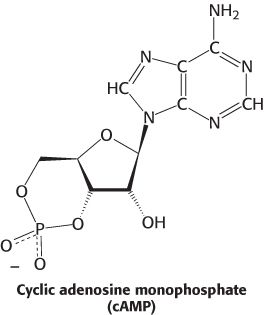
PKA provides a clear example of the integration of allosteric regulation and phosphorylation. PKA is activated by cAMP concentrations near 10 nM. The quaternary structure is reminiscent of that of ATCase. Like that enzyme, PKA in muscle consists of two kinds of subunits: a 49-
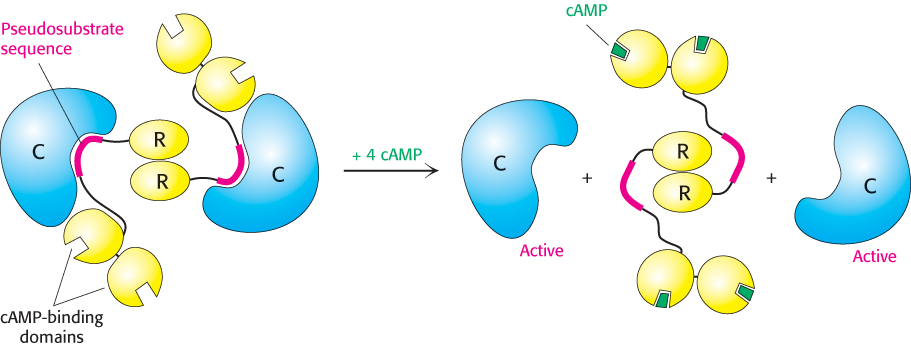
How does the binding of cAMP activate the kinase? Each R chain contains the sequence Arg-
298
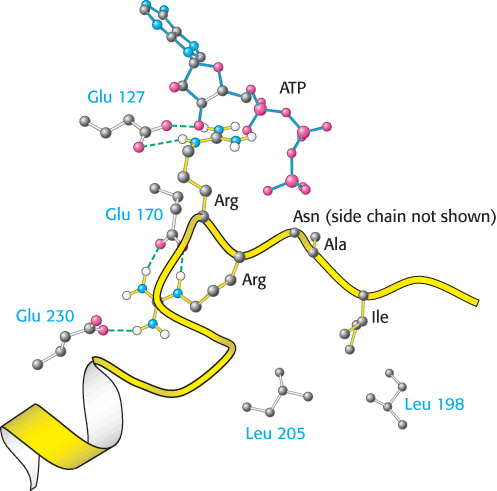
ATP and the target protein bind to a deep cleft in the catalytic subunit of protein kinase A
 X-
X-
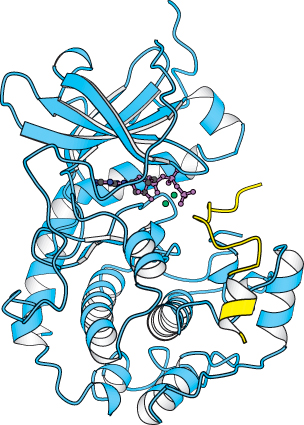
The bound peptide in this crystal occupies the active site because it contains the pseudosubstrate sequence Arg-
299