PROBLEMS
PROBLEMS
Question 10.1
Context please. The allosteric properties of aspartate transcarbamoylase have been discussed in detail in this chapter. What is the function of aspartate transcarbamoylase?
Question 10.2
Activity profile. A histidine residue in the active site of aspartate transcarbamoylase is thought to be important in stabilizing the transition state of the bound substrates. Predict the pH dependence of the catalytic rate, assuming that this interaction is essential and dominates the pH-
Question 10.3
Knowing when to say when. What is feedback inhibition? Why is it a useful property?
Question 10.4
Knowing when to get going. What is the biochemical rationale for ATP serving as a positive regulator of ATCase?
Question 10.5
No T. What would be the effect of a mutation in an allosteric enzyme that resulted in a T/R ratio of 0?
Question 10.6
Turned upside down. An allosteric enzyme that follows the concerted model has a T/R ratio of 300 in the absence of substrate. Suppose that a mutation reversed the ratio. How would this mutation affect the relation between the rate of the reaction and the substrate concentration?
Question 10.7
Partners. As shown in Figure 10.2, CTP inhibits ATCase; however, the inhibition is not complete. Can you suggest another molecule that might enhance the inhibition of ATCase? Hint: See Figure 25.2.
Question 10.8
RT equilibrium. Differentiate between homotropic and heterotropic effectors.
Question 10.9
Restoration project. If isolated regulatory subunits and catalytic subunits of ATCase are mixed, the native enzyme is reconstituted. What is the biological significance of the observation?
Question 10.10
Because it’s an enzyme. X-
Question 10.11
Allosteric switching. A substrate binds 100 times as tightly to the R state of an allosteric enzyme as to its T state. Assume that the concerted (MWC) model applies to this enzyme. (See equations for the Concerted Model in the Appendix to Chapter 7.)
By what factor does the binding of one substrate molecule per enzyme molecule alter the ratio of the concentrations of enzyme molecules in the R and T states?
Suppose that L, the ratio of [T] to [R] in the absence of substrate, is 107 and that the enzyme contains four binding sites for substrate. What is the ratio of enzyme molecules in the R state to those in the T state in the presence of saturating amounts of substrate, assuming that the concerted model is obeyed?
Question 10.12
Allosteric transition. Consider an allosteric protein that obeys the concerted model. Suppose that the ratio of T to R formed in the absence of ligand is 105, KT = 2 mM, and KR = 5 μM. The protein contains four binding sites for ligand. What is the fraction of molecules in the R form when 0, 1, 2, 3, and 4 ligands are bound? (See equations for the Concerted Model in the Appendix to Chapter 7.)
Question 10.13
Negative cooperativity. You have isolated a dimeric enzyme that contains two identical active sites. The binding of substrate to one active site decreases the substrate affinity of the other active site. Can the concerted model account for this negative cooperativity? Hint: See Section 7.2.
Question 10.14
A new view of cooperativity. Draw a double-
Question 10.15
Paradoxical at first glance. Recall that phosphonacetyl-
311
Question 10.16
Regulation energetics. The phosphorylation and dephosphorylation of proteins is a vital means of regulation. Protein kinases attach phosphoryl groups, whereas only a phosphatase will remove the phosphoryl group from the target protein. What is the energy cost of this means of covalent regulation?
Question 10.17
Vive la différence. What is an isozyme?
Question 10.18
Fine-
Question 10.19
Making matches.
|
(a) ATCase _________ |
1. Protein phosphorylation catalyst |
|
(b) T state _________ |
2. Required to modify glutamate |
|
(c) R state _________ |
3. Activates a particular kinase |
|
(d) Phosphorylation _________ |
4. Proenzyme |
|
(e) Kinase _________ |
5. Activates trypsin |
|
(f) Phosphatase _________ |
6. Common covalent modification |
|
(g) cAMP _________ |
7. Inhibited by CTP |
|
(h) Zymogen _________ |
8. Less- |
|
(i) Enteropeptidase _________ |
9. Initiates extrinsic pathway |
|
(j) Vitamin K _________ |
10. Forms fibrin |
|
(k) Thrombin _________ |
11. More- |
|
(l) Tissue factor _________ |
12. Removes phosphates |
Question 10.20
Powering change. Phosphorylation is a common covalent modification of proteins in all forms of life. What energetic advantages accrue from the use of ATP as the phosphoryl donor?
Question 10.21
No going back. What is the key difference between regulation by covalent modification and specific proteolytic cleavage?
Question 10.22
Zymogen activation. When very low concentrations of pepsinogen are added to acidic media, how does the half-
Question 10.23
No protein shakes advised. Predict the physiological effects of a mutation that resulted in a deficiency of enteropeptidase.
Question 10.24
A revealing assay. Suppose that you have just examined a young boy with a bleeding disorder highly suggestive of classic hemophilia (factor VIII deficiency). Because of the late hour, the laboratory that carries out specialized coagulation assays is closed. However, you happen to have a sample of blood from a classic hemophiliac whom you admitted to the hospital an hour earlier. What is the simplest and most rapid test that you can perform to determine whether your present patient also is deficient in factor VIII activity?
Question 10.25
Counterpoint. The synthesis of factor X, like that of prothrombin, requires vitamin K. Factor X also contains γ-carboxyglutamate residues in its amino-
Question 10.26
A discerning inhibitor. Antithrombin III forms an irreversible complex with thrombin but not with prothrombin. What is the most likely reason for this difference in reactivity?
Question 10.27
Drug design. A drug company has decided to use recombinant DNA methods to prepare a modified α1-anti-
Question 10.28
Blood must flow. Why is inappropriate blood-
Question 10.29
Hemostasis. Thrombin functions in both coagulation and fibrinolysis. Explain.
Question 10.30
Dissolution row. What is tissue-
Question 10.31
Joining together. What differentiates a soft clot from a mature clot?
Data Interpretation Problems
Question 10.32
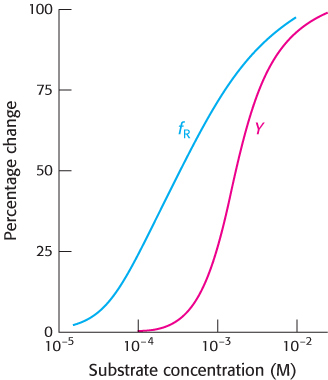
Distinguishing between models. The following graph shows the fraction of an allosteric enzyme in the R state (fR) and the fraction of active sites bound to substrate (Y) as a function of substrate concentration. Which model, the concerted or sequential, best explains these results?
312
Question 10.33
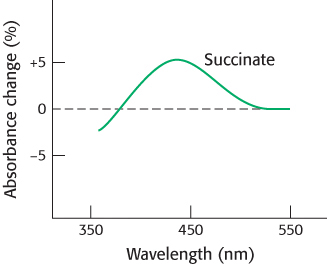
Reporting live from ATCase 1. ATCase underwent reaction with tetranitromethane to form a colored nitrotyrosine group (λmax = 430 nm) in each of its catalytic chains. The absorption by this reporter group depends on its immediate environment. An essential lysine residue at each catalytic site also was modified to block the binding of substrate. Catalytic trimers from this doubly modified enzyme were then combined with native trimers to form a hybrid enzyme. The absorption by the nitrotyrosine group was measured on addition of the substrate analog succinate. What is the significance of the alteration in the absorbance at 430 nm?
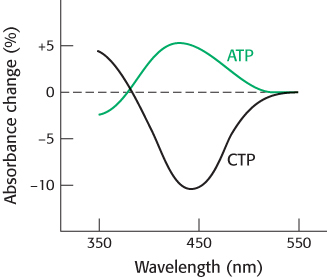
Question 10.34
Reporting live from ATCase 2. A different ATCase hybrid was constructed to test the effects of allosteric activators and inhibitors. Normal regulatory subunits were combined with nitrotyrosine-
Question 10.35
Conviviality and PKA. Recent studies have suggested that protein kinase A may be important in establishing behaviors in many organisms, including humans. One study investigated the role of PKA in locust behavior. Certain species of locust live solitary lives until crowded, at which point they become gregarious—
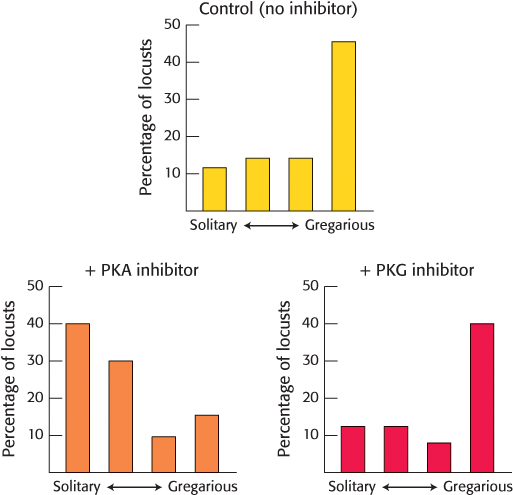
Locusts were grouped together for one hour, and then allowed to stay with the group or move away. Prior to crowding, some insects were injected with a PKA inhibitor, a cyclic GMP-
What is the response of the control group to crowding?
What is the result if the insects are first treated with PKA inhibitor? PKG inhibitor?
What was the purpose of the experiment with the PKG inhibitor?
313
What do these results suggest about the role of PKA in the transition from a solitary to a gregarious life style?
The above experiments were repeated on a different species of insect that is always gregarious. The results are shown below.
 [Data from S. R. Ott, et al., Proc. Natl. Acad. Sci. U. S. A. 109(7):E381–
[Data from S. R. Ott, et al., Proc. Natl. Acad. Sci. U. S. A. 109(7):E381–7, 2012.] What do these results suggest about the role of PKA in insects that are always gregarious?
Chapter Integration Problems
Question 10.36
Repeating heptads. Each of the three types of fibrin chains contains repeating heptapeptide units (abcdefg) in which residues a and d are hydrophobic. Propose a reason for this regularity.
Question 10.37
Density matters. The sedimentation value of aspartate transcarbamoylase decreases when the enzyme switches to the R state. On the basis of the allosteric properties of the enzyme, explain why the sedimentation value decreases.
Question 10.38
Too tight a grip. Trypsin cleaves proteins on the carboxyl side of lysine. Trypsin inhibitor has a lysine residue, and binds to trypsin, yet it is not a substrate. Explain.
Question 10.39
Apparently not the four-
Mechanism Problems
Question 10.40
Aspartate transcarbamoylase. Write the mechanism (in detail) for the conversion of aspartate and carbamoyl phosphate into N-carbamoylaspartate. Include a role for the histidine residue present in the active site.
Question 10.41
Protein kinases. Write a mechanism (in detail) for phosphorylation of a serine residue by ATP catalyzed by protein kinase. What groups might you expect to find in the enzyme’s active site?
314