PROBLEMS
PROBLEMS
Question 17.1
A one-
Question 17.2
Naming names. What are the five enzymes (including regulatory enzymes) that constitute the pyruvate dehydrogenase complex? Which reactions do they catalyze?
Question 17.3
Coenzymes. What coenzymes are required by the pyruvate dehydrogenase complex? What are their roles?
Question 17.4
More coenzymes. Distinguish between catalytic coenzymes and stoichiometric coenzymes in the pyruvate dehydrogenase complex.
Question 17.5
Waste and fraud? Figure 17.9 shows the steps in the pyruvate dehydrogenase complex reaction cycle. A key product, acetyl CoA, is released after the fourth step. What is the purpose of the remaining steps?
Question 17.6
Joined at the hip. List some of the advantages of organizing the enzymes that catalyze the formation of acetyl CoA from pyruvate into a single large complex.
Question 17.7
Flow of carbon atoms. What is the fate of the radioactive label when each of the following compounds is added to a cell extract containing the enzymes and cofactors of the glycolytic pathway, the citric acid cycle, and the pyruvate dehydrogenase complex? (The 14C label is printed in red.)
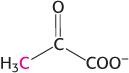
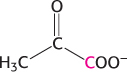
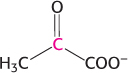
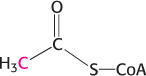
Glucose 6-
phosphate labeled at C- 1.
Question 17.8
C2 + C2 → C4
Which enzymes are required to get net synthesis of oxaloacetate from acetyl CoA?
Write a balanced equation for the net synthesis.
Do mammalian cells contain the requisite enzymes?
Question 17.9
Driving force. What is the ΔG°′ for the complete oxidation of the acetyl unit of acetyl CoA by the citric acid cycle?
Question 17.10
Acting catalytically. The citric acid cycle itself, which is composed of enzymatically catalyzed steps, can be thought of essentially as a supramolecular enzyme. Explain.
Question 17.11
A potent inhibitor. Thiamine thiazolone pyrophosphate binds to pyruvate dehydrogenase about 20,000 times as strongly as does thiamine pyrophosphate, and it competitively inhibits the enzyme. Why?
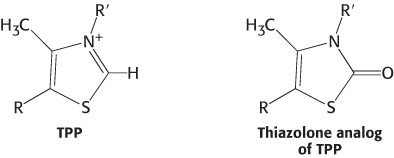
Question 17.12
Like Holmes and Watson. Match each term with its description.
|
(a) Acetyl CoA _____ (b) Citric acid cycle _____ (c) Pyruvate dehydrogenase complex _____ (d) Thiamine pyrophosphate _____ (e) Lipoic acid _____ (f) Pyruvate dehydrogenase _____ (g) Acetyllipoamide _____ (h) Dihydrolipoyl transacetylase _____ (i) Dihydrolipoyl dehydrogenase _____ (j) Beriberi _____ |
1. Catalyzes the link between glycolysis and the citric acid cycle. 2. Coenzyme required by transacetylase 3. Final product of pyruvate dehydrogenase 4. Catalyzes the formation of acetyl CoA 5. Regenerates active transacetylase 6. Fuel for the citric acid cycle 7. Coenzyme required by pyruvate dehydrogenase 8. Catalyzes the oxidative decarboxylation of pyruvate 9. Due to a deficiency of thiamine 10. Central metabolic hub |
Question 17.13
Lactic acidosis. Patients in shock often suffer from lactic acidosis owing to a deficiency of O2. Why does a lack of O2 lead to lactic acid accumulation? One treatment for shock is to administer dichloroacetate (DCA), which inhibits the kinase associated with the pyruvate dehydrogenase complex. What is the biochemical rationale for this treatment?
Question 17.14
DCA again. Patients with pyruvate dehydrogenase deficiency show high levels of lactic acid in the blood. However, in some cases, treatment with dichloroacetate (DCA) lowers lactic acid levels.
How does DCA act to stimulate pyruvate dehydrogenase activity?
What does this suggest about pyruvate dehydrogenase activity in patients who respond to DCA?
Question 17.15
Energy rich. What are the thioesters in the reaction catalyzed by PDH complex?
520
Question 17.16
Alternative fates. Compare the regulation of the pyruvate dehydrogenase complex in muscle and in liver.
Question 17.17
Mutations.
Predict the effect of a mutation that enhances the activity of the kinase associated with the PDH complex.
Predict the effect of a mutation that reduces the activity of the phosphatase associated with the PDH complex.
Question 17.18
Flaking paint, green wallpaper. Clare Boothe Luce, ambassador to Italy in the 1950s (and Connecticut congressperson, playwright, editor of Vanity Fair, and the wife of Henry Luce, founder of Time magazine and Sports Illustrated) became ill when she was staying at the ambassadorial residence in Rome. The paint on the dining room ceiling, an arsenic-
Question 17.19
Like Jack and Jill. Match each enzyme with its description.
|
(a) Pyruvate dehydrogenase complex _____ (b) Citrate synthase _____ (c) Aconitase _____ (d) Isocitrate dehydrogenase _____ (e) α-Ketoglutarate dehydrogenase _____ (f) Succinyl CoA synthetase _____ (g) Succinate dehydrogenase _____ (h) Fumarase _____ (i) Malate dehydrogenase _____ (j) Pyruvate carboxylase _____ |
1. Catalyzes the formation of isocitrate 2. Synthesizes succinyl CoA 3. Generates malate 4. Generates ATP 5. Converts pyruvate into acetyl CoA 6. Converts pyruvate into oxaloacetate 7. Condenses oxaloacetate and acetyl CoA 8. Catalyzes the formation of oxaloacetate 9. Synthesizes fumarate 10. Catalyzes the formation of α-ketoglutarate |
Question 17.20
A hoax, perhaps? The citric acid cycle is part of aerobic respiration, but no O2 is required for the cycle. Explain this paradox.
Question 17.21
One of a kind. How is succinate dehydrogenase unique compared with the other enzymes in the citric acid cycle?
Question 17.22
Coupling reactions. The oxidation of malate by NAD+ to form oxaloacetate is a highly endergonic reaction under standard conditions [ΔG°′ = 29 kJ mol−1 (7 kcal mol−1)]. The reaction proceeds readily under physiological conditions.
Explain why the reaction proceeds as written under physiological conditions.
Assuming an [NAD+]/[NADH] ratio of 8 and a pH of 7, what is the lowest [malate]/[oxaloacetate] ratio at which oxaloacetate can be formed from malate?
Question 17.23
Synthesizing α-ketoglutarate. It is possible, with the use of the reactions and enzymes considered in this chapter, to convert pyruvate into α-ketoglutarate without depleting any of the citric acid cycle components. Write a balanced reaction scheme for this conversion, showing cofactors and identifying the required enzymes.
Question 17.24
Seven o’clock roadblock. Malonate is a competitive inhibitor of succinate dehydrogenase. How will the concentrations of citric acid cycle intermediates change immediately after the addition of malonate? Why is malonate not a substrate for succinate dehydrogenase?
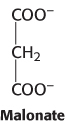
Question 17.25
No signal, no activity. Why is acetyl CoA an especially appropriate activator for pyruvate carboxylase?
Question 17.26
Power differentials. As we will see in the next chapter, when NADH reacts with oxygen, 2.5 ATP are generated. When FADH2 reduces oxygen, only 1.5 ATP are generated. Why then does succinate dehydrogenase produce FADH2 and not NADH when succinate is reduced to fumarate?
Question 17.27
Back to Orgo (or do you say O chem?). Before any oxidation can occur in the citric acid cycle, citrate must be isomerized into isocitrate. Why is this the case?
Question 17.28
A nod is as good as a wink to a blind horse. Explain why a GTP molecule, or another nucleoside triphosphate, is energetically equivalent to an ATP molecule in metabolism.
Question 17.29
One from two. The synthesis of citrate from acetyl CoA and oxaloacetate is a biosynthetic reaction. What is the energy source that drives formation of citrate?
Question 17.30
Versatility. What is the chief benefit of being able to perform the glyoxylate cycle?
Chapter Integration Problems
Question 17.31
Fats into glucose? Fats are usually metabolized into acetyl CoA and then further processed through the citric acid cycle. In Chapter 16, we saw that glucose can be synthesized from oxaloacetate, a citric acid cycle intermediate. Why, then, after a long bout of exercise depletes our carbohydrate stores, do we need to replenish those stores by eating carbohydrates? Why do we not simply replace them by converting fats into carbohydrates?
521
Question 17.32
Alternative fuels. As we will see (Chapter 22), fatty acid breakdown generates a large amount of acetyl CoA. What will be the effect of fatty acid breakdown on pyruvate dehydrogenase complex activity? On glycolysis?
Mechanism Problems
Question 17.33
Theme and variation. Propose a reaction mechanism for the condensation of acetyl CoA and glyoxylate in the glyoxylate cycle of plants and bacteria.
Question 17.34
Symmetry problems. In experiments carried out in 1941 to investigate the citric acid cycle, oxaloacetate labeled with 14C in the carboxyl carbon atom farthest from the keto group was introduced to an active preparation of mitochondria.
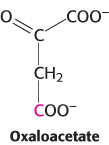
Analysis of the α-ketoglutarate formed showed that none of the radioactive label had been lost. Decarboxylation of α-ketoglutarate then yielded succinate devoid of radioactivity. All the label was in the released CO2. Why were the early investigators of the citric acid cycle surprised that all the label emerged in the CO2?
Question 17.35
Symmetric molecules reacting asymmetrically. The interpretation of the experiments described in Problem 34 was that citrate (or any other symmetric compound) cannot be an intermediate in the formation of α-ketoglutarate, because of the asymmetric fate of the label. This view seemed compelling until Alexander Ogston incisively pointed out in 1948 that “it is possible that an asymmetric enzyme which attacks a symmetrical compound can distinguish between its identical groups [italics added].” For simplicity, consider a molecule in which two hydrogen atoms, a group X, and a different group Y are bonded to a tetrahedral carbon atom as a model for citrate. Explain how a symmetric molecule can react with an enzyme in an asymmetric way.
Data Interpretation Problem
Question 17.36
A little goes a long way. As will become clearer in Chapter 18, the activity of the citric acid cycle can be monitored by measuring the amount of O2 consumed. The greater the rate of O2 consumption, the faster the rate of the cycle. Hans Krebs used this assay to investigate the cycle in 1937. He used as his experimental system minced pigeon-
Effect of citrate on oxygen consumption by minced pigeon-
|
Micromoles of oxygen consumed |
||
|---|---|---|
|
Time (min) |
Carbohydrate only |
Carbohydrate plus 3 μmol of citrate |
|
10 |
26 |
28 |
|
60 |
43 |
62 |
|
90 |
46 |
77 |
|
150 |
49 |
85 |
How much O2 would be absorbed if the added citrate were completely oxidized to H2O and CO2?
On the basis of your answer to part a, what do the results given in the table suggest?
Question 17.37
Arsenite poisoning. The effect of arsenite on the experimental system of Problem 36 was then examined. Experimental data (not presented here) showed that the amount of citrate present did not change in the course of the experiment in the absence of arsenite. However, if arsenite was added to the system, different results were obtained, as shown in the following table.
Disappearance of citric acid in pigeon-
|
Micromoles of citrate added |
Micromoles of citrate found after 40 minutes |
Micromoles of citrate used |
|---|---|---|
|
22 |
0.6 |
21 |
|
44 |
20.0 |
24 |
|
90 |
56.0 |
34 |
What is the effect of arsenite on the disappearance of citrate?
How is the action of arsenite altered by the addition of more citrate?
What do these data suggest about the site of action of arsenite?
Question 17.38
Isocitrate lyase and tuberculosis. The bacterium Mycobacterium tuberculosis, the cause of tuberculosis, can invade the lungs and persist in a latent state for years. During this time, the bacteria reside in granulomas—
522
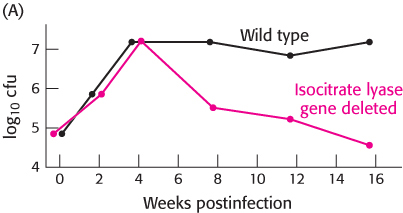
In graph A, the black circles represent the results for wild-
What is the effect of the absence of isocitrate lyase?
The techniques described in Chapter 5 were used to reinsert the gene encoding isocitrate lyase into bacteria from which it had previously been deleted.
In graph B, black circles represent bacteria into which the gene was reinserted and red circles represent bacteria in which the gene was still missing.
Do these results support those obtained in part a?
What is the purpose of the experiment in part b?
Why do these bacteria perish in the absence of the glyoxylate cycle?
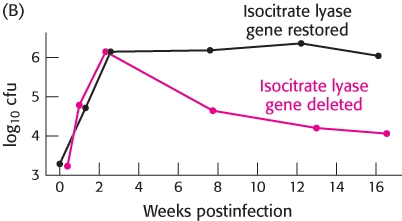 [Data from KcKinney et al., Nature 406:735–
[Data from KcKinney et al., Nature 406:735–738, 2000.]