19.4A Proton Gradient across the Thylakoid Membrane Drives ATP Synthesis
A Proton Gradient across the Thylakoid Membrane Drives ATP Synthesis
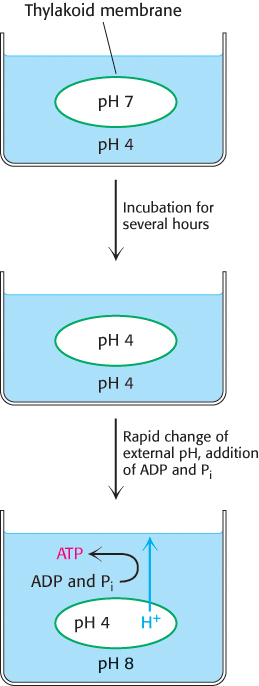
In 1966, André Jagendorf showed that chloroplasts synthesize ATP in the dark when an artificial pH gradient is imposed across the thylakoid membrane. To create this transient pH gradient, he soaked chloroplasts in a pH 4 buffer for several hours and then rapidly mixed them with a pH 8 buffer containing ADP and Pi. The pH of the stroma suddenly increased to 8, whereas the pH of the thylakoid space remained at 4. A burst of ATP synthesis then accompanied the disappearance of the pH gradient across the thylakoid membrane (Figure 19.24). This incisive experiment was one of the first to unequivocally support the hypothesis put forth by Peter Mitchell that ATP synthesis is driven by proton-
The principles of ATP synthesis in chloroplasts are nearly identical with those in mitochondria. ATP formation is driven by a proton-
The ATP synthase of chloroplasts closely resembles those of mitochondria and prokaryotes
The proton-
CF0 is embedded in the thylakoid membrane. It consists of four different polypeptide chains known as I (17 kDa), II (16.5 kDa), III (8 kDa), and IV (27 kDa) having an estimated stoichiometry of 1 : 2 : 10–
579
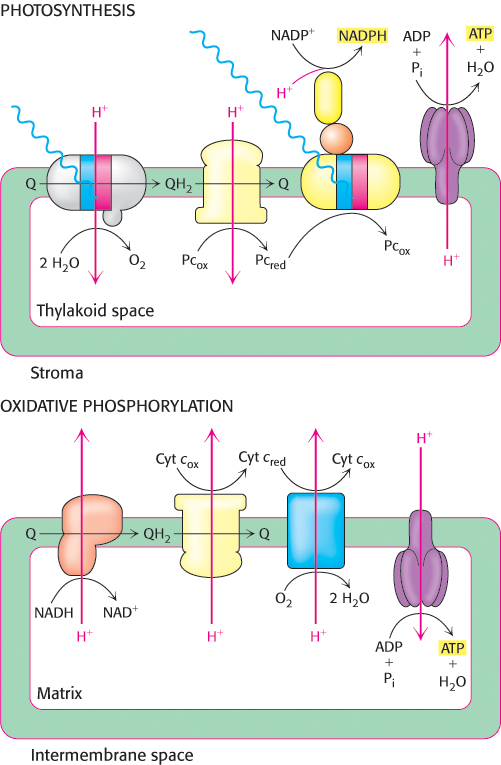
Note that the membrane orientation of CF1–CF0 is reversed compared with that of the mitochondrial ATP synthase (Figure 19.25). However, the functional orientation of the two synthases is identical: protons flow from the lumen through the enzyme to the stroma or matrix where ATP is synthesized. Because CF1 is on the stromal surface of the thylakoid membrane, the newly synthesized ATP is released directly into the stromal space. Likewise, NADPH formed by photosystem I is released into the stromal space. Thus, ATP and NADPH, the products of the light reactions of photosynthesis, are appropriately positioned for the subsequent dark reactions, in which CO2 is converted into carbohydrate.
The activity of chloroplast ATP synthase is regulated
The activity of the ATP synthase is sensitive to the redox conditions in the chloroplast. For maximal activity, a specific disulfide bond in the γ subunit must be reduced to two cysteines. The reductant is reduced thioredoxin, which is formed from ferredoxin generated in photosystem I, by ferredoxin–
580
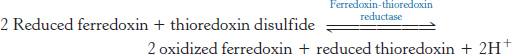
Conformational changes in the ϵ subunit also contribute to synthase regulation. The ϵ subunit appears to exist in two conformations. One conformation inhibits ATP hydrolysis by the synthase, while the other, which is generated by an increase in the proton-
Cyclic electron flow through photosystem I leads to the production of ATP instead of NADPH
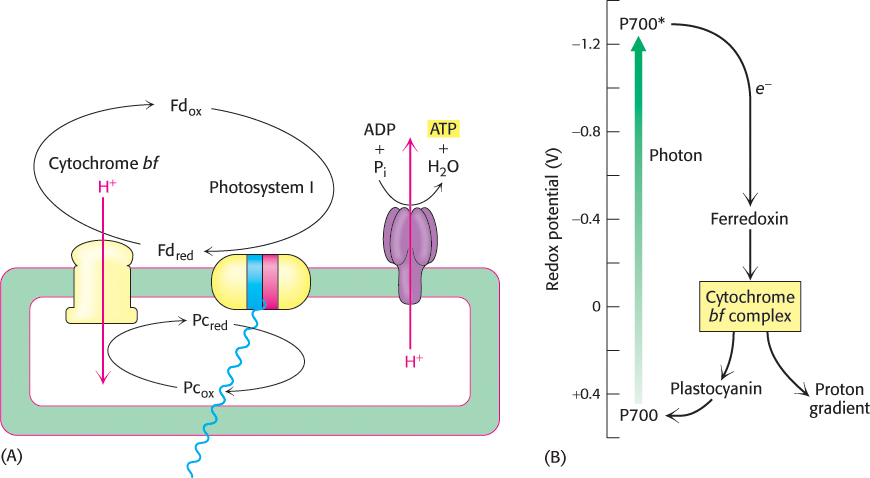
On occasion, when the ratio of NADPH to NADP+ is very high, as might be the case if there was another source of electrons to form NADPH (Section 20.3), NADP+ may be unavailable to accept electrons from reduced ferredoxin. In these circumstances, specific large protein complexes allow cyclic electron flow that powers ATP synthesis. Electrons arising from P700, the reaction center of photosystem I, generate reduced ferredoxin. The electron in reduced ferredoxin is transferred to the cytochrome bf complex rather than to NADP+. This electron then flows back through the cytochrome bf complex to reduce plastocyanin, which can then be reoxidized by P700+ to complete a cycle. The net outcome of this cyclic flow of electrons is the pumping of protons by the cytochrome bf complex. The resulting proton gradient then drives the synthesis of ATP. In this process, called cyclic photophosphorylation, ATP is generated without the concomitant formation of NADPH (Figure 19.26). Photosystem II does not participate in cyclic photophosphorylation, and so O2 is not formed from H2O.
581
The absorption of eight photons yields one O2, two NADPH, and three ATP molecules
We can now estimate the overall stoichiometry for the light reactions. The absorption of four photons by photosystem II generates one molecule of O2 and releases four protons into the thylakoid lumen. The two molecules of plastoquinol are oxidized by the Q cycle of the cytochrome bf complex to release eight protons into the lumen. Finally, the electrons from four molecules of reduced plastocyanin are driven to ferredoxin by the absorption of four additional photons. The four molecules of reduced ferredoxin generate two molecules of NADPH. Thus, the overall reaction is
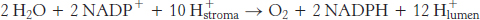
The 12 protons released in the lumen can then flow through ATP synthase. Let us assume that there are 12 subunit III components in CF0. We expect that 12 protons must pass through CF0 to complete one full rotation of CF1. A single rotation generates three molecules of ATP. Given the ratio of 3 ATP for 12 protons, the overall reaction is
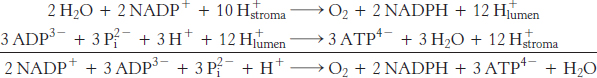
Thus, eight photons are required to yield three molecules of ATP (2.7 photons/ATP).
Cyclic photophosphorylation is a somewhat more productive way to synthesize ATP than noncyclic photophosphorylation (the Z scheme). The absorption of four photons by photosystem I leads to the release of eight protons into the lumen by the cytochrome bf system. These protons flow through ATP synthase to yield two molecules of ATP. Thus, each two absorbed photons yield one molecule of ATP. No NADPH is produced.