27.2
The Brain Plays a Key Role in Caloric Homeostasis
What makes this remarkable balance of energy input and output possible? As one might imagine, the answer is complicated, entailing many biochemical signals as well as a host of behavioral factors. We will focus on a few key biochemical signals, and divide our discussion into two parts: short-term signals that are active during a meal and long-term signals that report on the overall energy status of the body. These signals originate in the gastrointestinal tract, the β cells of the pancreas, and fat cells. The primary target of these signals is the brain, in particular a group of neurons in a region of the hypothalamus called the arcuate nucleus.
Signals from the gastrointestinal tract induce feelings of satiety
Short-term signals relay feelings of satiety from the gut to various regions of the brain and thus reduce the urge to eat (Figure 27.2). The best-studied short-term signal is cholecystokinin (CCK). Cholecystokinin is actually a family of peptide hormones of various lengths (from 8 to 58 amino acids in length, depending on posttranslational processing) secreted into the blood by cells in the duodenum and jejunum regions of the small intestine as a postprandial satiation signal. The CCK binds to the CCK receptor, a G-protein-coupled receptor (Section 14.1) located in various peripheral neurons, that relay signals to the brain. This binding initiates a signal-transduction pathway in the brain that generates a feeling of satiety. CCK also plays an important role in digestion, stimulating the secretion of pancreatic enzymes and bile salts from the gallbladder.
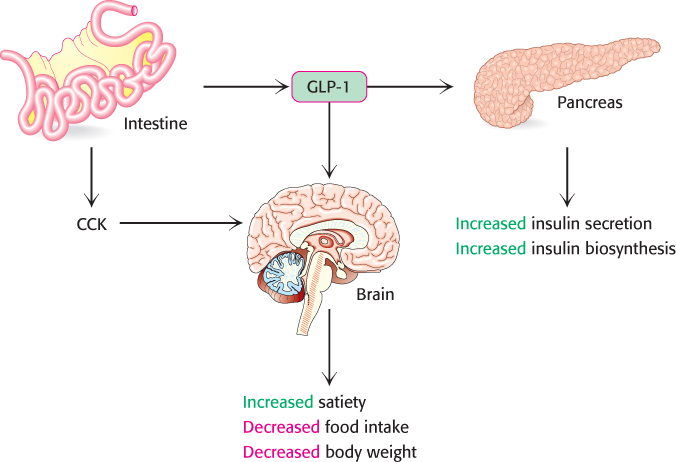
FIGURE 27.2Satiation signals. Cholecystokinin (CCK) and glucagon-like peptide 1 (GLP-1) are signal molecules that induce feelings of satiety in the brain. CCK is secreted by specialized cells of the small intestine in response to a meal and activates satiation pathways in the brain. GLP-1, secreted by L cells in the intestine, also activates satiation pathways in the brain and potentiates insulin action in the pancreas.
[Information from S. C. Wood, Cell Metab. 9:489–498, 2009, Fig. 1.]
Another important gut signal is glucagon-like peptide 1 (GLP-1), a hormone of approximately 30 amino acids in length. GLP-1 is secreted by intestinal L cells, hormone-secreting cells located throughout the lining of the gastrointestinal tract. GLP-1 has a variety of effects, all apparently facilitated by binding to its receptor, another G-protein-coupled receptor. Like CCK, GLP-1 induces feelings of satiety that inhibit further eating. GLP-1 also potentiates glucose-induced insulin secretion by the β cells of the pancreas while inhibiting glucagon secretion.
Although we have examined only two short-term signals, many others are believed to exist (Table 27.2). Most of the short-term signals thus far identified are appetite suppressants. Ghrelin, a peptide that is 28 amino acids in length and secreted by the stomach, acts on regions of the hypothalamus to stimulate appetite through its receptor, a G-protein-coupled receptor. Ghrelin secretion increases before a meal and decreases afterward.
TABLE 27.2 Gastrointestinal peptides that regulate food intake
Appetite-suppressing signals |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gastric inhibitory peptide |
| |
Appetite-enhancing peptides |
|
|
Information from: M. H. Stipanuk, ed., Biochemical, Physiological, Molecular Aspects of Human Nutrition, 2d ed. (Saunders/Elsevier, 2006), p. 627, Box 22-1. |
Leptin and insulin regulate long-term control over caloric homeostasis
Two key signal molecules regulate energy homeostasis over the time scale of hours or days: leptin, which is secreted by the adipocytes, and insulin, which is secreted by the β cells of the pancreas. Leptin reports on the status of the triacylglycerol stores, whereas insulin reports on the status of glucose in the blood—in other words, of carbohydrate availability. We will consider leptin first.
Adipose tissue was formerly considered an inert depot of triacylglycerols. However, recent work has shown that adipose tissue is an active endocrine tissue, secreting signal molecules called adipokines, such as leptin, that regulate a host of physiological processes. Leptin is secreted by the adipocytes in direct proportion to the amount of fat present. The more fat in a body, the more leptin is secreted. Leptin binding to its receptor throughout the body increases the sensitivity of muscle and the liver to insulin, stimulates β oxidation of fatty acids, and decreases triacylglycerol synthesis.
Let us consider the effects of leptin in the brain. Leptin binds to its receptor, thereby activating a signal transduction pathway. The leptin receptor is found in various regions of the brain, but particularly in the arcuate nucleus of the hypothalamus. There, one population of neurons expresses appetite-stimulating (orexigenic) peptides, called neuropeptide Y (NPY) and agouti-related peptide (AgRP). Fasting stimulates the production of NPY and AgRP owing to the decrease in leptin levels that results from diminishing adipose tissue (Figure 27.3A). Leptin, on the other hand, inhibits the NPY/AgRP neurons, preventing the release of NPY and AgRP and thus repressing the desire to eat (Figure 27.3B).
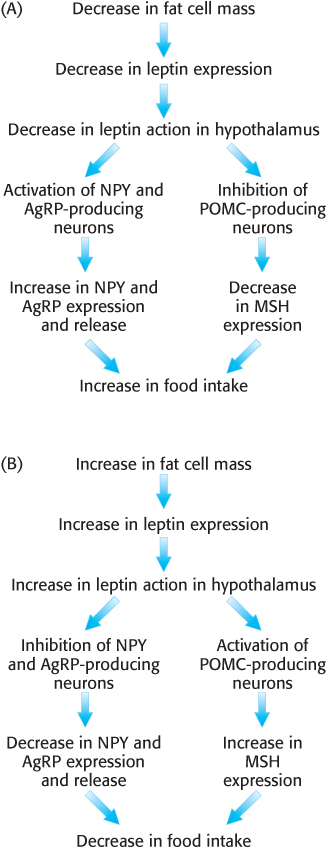
FIGURE 27.3The effects of leptin in the brain. Leptin is an adipokine secreted by adipose tissue in direct relation to fat mass. (A) When leptin levels fall, as in fasting, appetite-enhancing neuropeptides NPY and AgRP are secreted, whereas the secretion of appetite-suppressing signals such as MSH is inhibited. (B) When fat mass increases, leptin inhibits NPY and AgRP secretion while stimulating the release of appetite-suppressing hormone MSH.
[Information from M. H. Stipanuk, Biochemical, Physiological, & Molecular Aspects of Human Nutrition, 2d ed. (Saunders/Elsevier, 2006), Fig. 22-2.]
The second population of neurons containing leptin receptors expresses a large precursor polypeptide, proopiomelanocortin (POMC). In response to leptin binding to its receptor on POMC neurons, POMC is proteolytically processed to yield a variety of signal molecules, one of which, melanocyte-stimulating hormone (MSH), is especially important in this context. MSH, originally discovered as a stimulator of melanocytes (cells that synthesize the pigment melanin), activates appetite-suppressing (anorexigenic) neurons and thus inhibits food consumption. Fasting inhibits MSH activity and thus stimulates eating. AgRP inhibits MSH activity by acting as an antagonist, binding to the MSH receptor but failing to activate the receptor (Figure 27.3). Thus, the net effect of leptin binding to its receptor is the initiation of a complex signal-transduction pathway that ultimately curtails food intake.
Insulin receptors are also present in the hypothalamus, although the mechanism of insulin action in the brain is less clear than that of leptin. Insulin appears to inhibit NPY/AgRP-producing neurons, thus inhibiting food consumption.
Leptin is one of several hormones secreted by adipose tissue
Leptin was the first adipokine discovered because of the dramatic effects of its absence. Researchers discovered a strain of mice called ob/ob mice, which lack leptin and, as a result, are extremely obese (refer to Chapter 22 opening figure). These mice display hyperphagia (overeating), hyperlipidemia (accumulation of triacylglycerols in muscle and liver), and an insensitivity to insulin. Since the discovery of leptin, other adipokines have been detected. For instance, adiponectin is another signal molecule produced by the adipocytes. Secretion of adiponectin falls in direct proportion to increases in fat mass. A key function of adiponectin appears to be increasing the sensitivity of the organism to insulin. Both leptin and adiponectin exert their effects through the key regulatory enzyme, AMP-activated protein kinase (AMPK). Recall that this enzyme is active when AMP levels are elevated and ATP levels are diminished, and this activation leads to a decrease in anabolism and an increase in catabolism, most notably an increase in fatty acid oxidation (Section 22.6). In insulin-resistant obese animals such as the ob/ob mice, leptin levels increase while those of adiponectin decrease.
Adipocytes also produce two hormones, RBP4 (originally discovered as a retinol binding protein) and resistin, that promote insulin resistance. Although it is unclear why adipocytes secrete hormones that facilitate insulin resistance, a pathological condition, we can speculate on the reason. These signal molecules may help to fine-tune the actions of leptin and adiponectin or perhaps to act as “brakes” on the action of leptin and adiponectin to prevent hypoglycemia in the fasted state. Some evidence indicates that enlarged adipocytes that result from obesity may secrete higher levels of insulin-antagonizing hormones and thus contribute to insulin resistance. Resistin has recently been implicated as a causal factor in the increased incidence of cardiovascular disease in obese individuals.
Leptin resistance may be a contributing factor to obesity
If leptin is produced in proportion to body-fat mass and leptin inhibits eating, why do people become obese? Obese people, in most cases, have both functioning leptin receptors and a high blood concentration of leptin. The failure to respond to the anorexigenic effects of leptin is called leptin resistance. What is the basis of leptin resistance?
As for most questions in the exciting area of energy homeostasis, the answer is not well worked out, but recent evidence suggests that a group of proteins called suppressors of cytokine signaling (SOCS) may take part. These proteins fine-tune some hormonal systems by inhibiting receptor action. SOCS proteins inhibit receptor signaling by a number of means. Consider, for example, the effect of SOCS proteins on the insulin receptor. Recall that insulin stimulates the autophosphorylation of tyrosine residues on the insulin receptor, which in turn phosphorylates IRS-1, initiating the insulin-signaling pathway (Figure 27.4A). SOCS proteins bind to phosphorylated tyrosine residues on receptors or other members of the signal-transduction pathway, thereby disrupting signal flow and thus altering the cell’s biochemical activity (Figure 27.4B). In other cases, the binding of SOCS proteins to components of the signal-transduction pathway may also enhance proteolytic degradation of these components by the proteasome (Section 23.2). Evidence in support of a role for SOCS in leptin resistance comes from mice that have had SOCS selectively deleted from POMC-expressing neurons. These mice display an enhanced sensitivity to leptin and are resistant to weight gain even when fed a high-fat diet. The reason why the activity of SOCS proteins increases, leading to leptin resistance, remains to be determined.
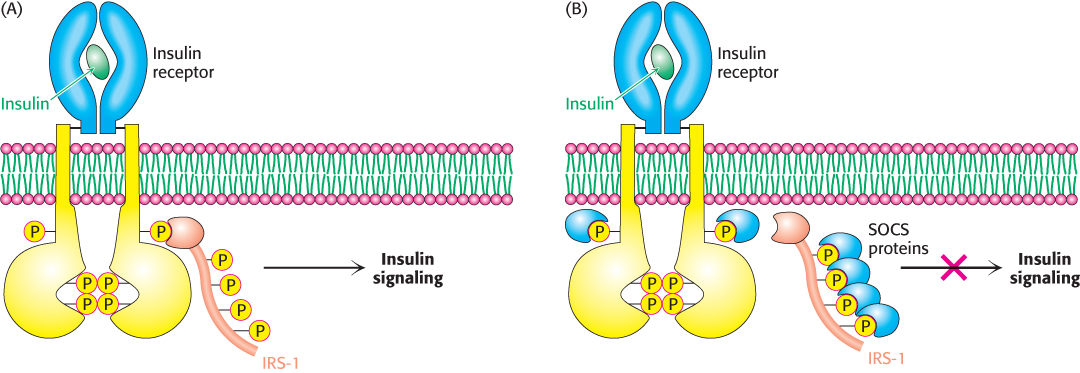
FIGURE 27.4Suppressors of cytokine signaling (SOCS) regulate receptor function. (A) Insulin binding results in phosphorylation of the receptor and subsequent phosphorylation of IRS-1. These processes initiate the insulin-signaling pathway. (B) SOCS proteins disrupt interactions of components of the insulin-signaling pathway by binding phosphorylated proteins and thereby inhibiting the pathway. The binding of a signal component by SOCS results in proteasomal degradation in some cases. (IRS-1, insulin-receptor substrate 1; SOCS, suppressor of cytokine signaling.)
Dieting is used to combat obesity
Given the obesity epidemic that we currently face and its associated disorders, much attention has been focused on determining the most effective weight-loss diet. In general, two categories of diet try to help us control our caloric intake—low-carbohydrate diets and low-fat diets. Low-carbohydrate diets usually emphasize protein consumption. Although studies of the effects of diets on humans are immensely complex, data are beginning to accumulate suggesting that low-carbohydrate–high-protein diets may be the most effective for losing weight. The exact reasons are not clear, but there are two common hypotheses. First, proteins seem to induce a feeling of satiation more effectively than do fats or carbohydrates. Second, proteins require more energy to digest than do fats or carbohydrates, and the increased energy expenditure contributes to weight loss. For instance, some studies show that a diet that is 30% protein requires almost 30% more energy to digest than that required by a diet that is 10% protein. The mechanisms by which protein-rich diets enhance energy expenditure and feelings of satiation remain to be determined. Regardless of the type of diet, the adage “Eat less, exercise more” always applies.


