35.1
Most Molecular-Motor Proteins Are Members of the P-Loop NTPase Superfamily
Eukaryotic cells contain three major families of motor proteins: myosins, kinesins, and dyneins. Members of each of these classes move along components of the cytoskeleton, but, at first glance, these protein families appear to be quite different from one another. Myosin, first characterized on the basis of its role in muscle, moves along filaments of the protein actin. Each molecule of muscle myosin consists of two copies each of a heavy chain with a molecular mass of 220 kDa, an essential light chain, and a regulatory light chain. The human genome encodes more than 40 distinct myosins; some function in muscle contraction, and others participate in a variety of other processes. Kinesins, which have roles in protein, mRNA, and vesicle transport as well as construction of the mitotic spindle and chromosome segregation, are generally dimers of two polypeptides. The human genome encodes more than 40 kinesins. Dyneins power the motion of cilia and flagella, and a general cytoplasmic dynein contributes to a variety of motions in all cells, including vesicle transport and various transport events in mitosis. Dyneins are enormous, with heavy chains of molecular mass greater than 500 kDa. The human genome encodes approximately 10 dyneins.
Initially, comparison of the amino acid sequences of myosins, kinesins, and dyneins did not reveal significant relationships between these protein families but, after their three-dimensional structures were determined, members of the myosin and kinesin families were found to have remarkable similarities. In particular, both myosin and kinesin contain P-loop NTPase cores homologous to those found in G proteins. Sequence analysis of the dynein heavy chain reveals it to be a member of the AAA subfamily of P-loop NTPases that we encountered in the context of the 19S proteasome (Section 23.2). Dynein has six sequences encoding such P-loop NTPase domains arrayed along its length, although only four actually appear to bind nucleotides. Thus, we expect similarities in the mechanisms of action, and we can draw on our knowledge of P-loop NTPases in general as we analyze the mechanisms of action of these motor proteins.
Molecular motors are generally oligomeric proteins with an ATPase core and an extended structure
Let us first consider the structure of myosin, which we examined briefly in Chapter 9. The results of electron microscopic studies of skeletal-muscle myosin show it to be a two-headed structure linked to a long stalk (Figure 9.42). The treatment of myosin with trypsin and papain results in the formation of four fragments: two S1 fragments; heavy meromyosin (HMM) which consists of the S1 fragments and an additional region termed S2; and a fragment called light meromyosin (LMM; Figure 35.2). Each S1 fragment corresponds to one of the heads of the intact structure and includes 850 amino-terminal amino acids from one of the two heavy chains as well as one copy of each of the light chains. Examination of the structure of an S1 fragment at high resolution reveals the presence of the P-loop NTPase-domain core that is the site of ATP binding and hydrolysis (Figure 35.3). We examined the structure and mechanism of action of this motor domain in Chapter 9.
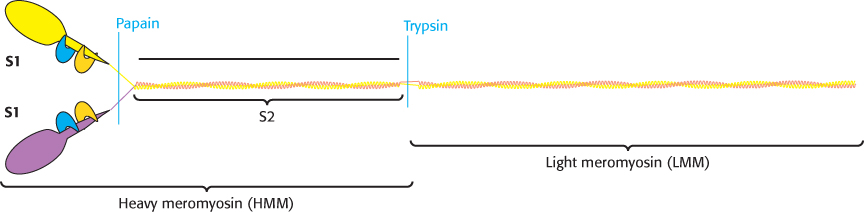
FIGURE 35.2Myosin dissection. Treatment of muscle myosin with proteases forms stable fragments, including subfragments S1 and S2 and light meromyosin. Each S1 fragment includes a head (shown in yellow or purple) from the heavy chain and one copy of each light chain (shown in blue and orange).
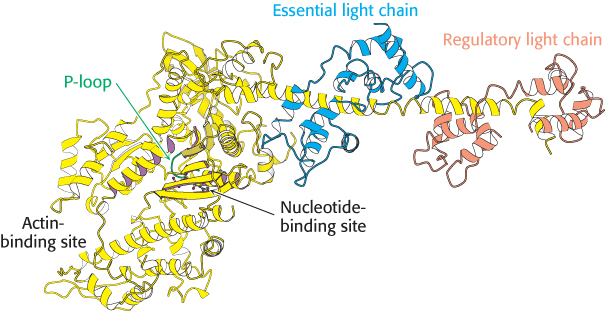
 FIGURE 35.3 Myosin structure at high resolution. The structure of the S1 fragment from muscle myosin reveals the presence of a P-loop NTPase domain (shaded in purple). Notice that an α helix extending from this domain is the binding site for the two light chains.
FIGURE 35.3 Myosin structure at high resolution. The structure of the S1 fragment from muscle myosin reveals the presence of a P-loop NTPase domain (shaded in purple). Notice that an α helix extending from this domain is the binding site for the two light chains.
[Drawn from 1DFL.pdb.]
Extending away from this structure is a long α helix from the heavy chain. This helix is the binding site for the two light chains. The light chains are members of the EF-hand family, similar to calmodulin (Figure 14.17), although most of the EF hands in light chains do not bind metal ions (Figure 35.4). Like calmodulin, these proteins wrap around an α helix, serving to thicken and stiffen it. The remaining fragments of myosin—S2 and light meromyosin—are largely α helical, forming two-stranded coiled coils created by the remaining lengths of the two heavy chains wrapping around each other (Figure 35.5). These structures, together extending approximately 1700 Å, link the myosin heads to other structures. In muscle myosin, several LMM domains come together to form higher-order bundles.

 FIGURE 35.5 Myosin two-stranded coiled coil. The two α helices form left-handed supercoiled structures that spiral around each other. Such structures are stabilized by hydrophobic residues at the contact points between the two helices.
FIGURE 35.5 Myosin two-stranded coiled coil. The two α helices form left-handed supercoiled structures that spiral around each other. Such structures are stabilized by hydrophobic residues at the contact points between the two helices.
[Drawn from 2TMA.pdb.]
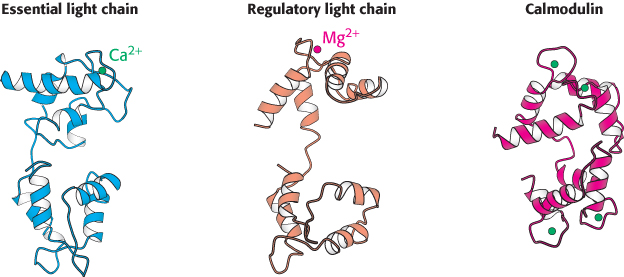
 FIGURE 35.4 Myosin light chains. The structures of the essential and regulatory light chains of muscle myosin are compared with the structure of calmodulin. Notice the similarities in the structures that allow each of these homologous proteins to bind an α helix (not shown) by wrapping around it.
FIGURE 35.4 Myosin light chains. The structures of the essential and regulatory light chains of muscle myosin are compared with the structure of calmodulin. Notice the similarities in the structures that allow each of these homologous proteins to bind an α helix (not shown) by wrapping around it.
[Drawn from 1DFL.pdb and 1CM1.pdb.]
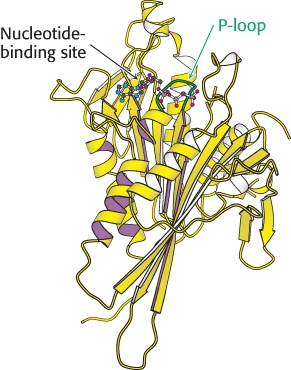
 FIGURE 35.7 Structure of head domain of kinesin at high resolution. Notice that the head domain of kinesin has the structure of a P-loop NTPase core (indicated by purple shading).
FIGURE 35.7 Structure of head domain of kinesin at high resolution. Notice that the head domain of kinesin has the structure of a P-loop NTPase core (indicated by purple shading).
[Drawn from 1I6I.pdb.]
Conventional kinesin (kinesin 1), the first kinesin discovered, has several structural features in common with myosin. The dimeric protein has two heads connected by an extended structure (Figure 35.6). The size of the head domain is approximately one-third that of myosin. Determination of the three-dimensional structure of a kinesin fragment revealed that the head domain also is built around a P-loop NTPase core (Figure 35.7). The myosin domain is so much larger than that of kinesin because of two large insertions in the myosin domain that bind to actin filaments. For conventional kinesin, a region of approximately 500 amino acids extends from the head domain. Like the corresponding region in myosin, the extended part of kinesin forms an α-helical coiled coil. Conventional kinesin also has light chains, but, unlike those of myosin, these light chains bind near the carboxyl terminus of the heavy chain and are thought to link the motor to intracellular cargo.
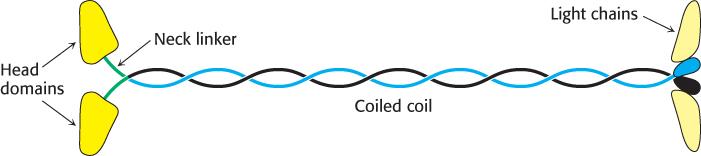
FIGURE 35.6Structure of kinesin. The elongated structure has the head domains at one end and the cargo-binding domains at the other, linked by a long coiled-coil region.
Dynein has a significantly different structure than myosin or kinesin. As noted earlier, the dynein heavy chain includes six regions that are homologous to the AAA subfamily of ATPase domains. A model for the structure of the motor domain of dynein was generated based on the structures of other AAA ATPases. This model has been confirmed and extended by more recent structure determinations of dynein itself (Figure 35.8). The head domain is appended to a region of approximately 1300 amino acids that forms an extended structure that links dynein units together to form oligomers and interacts with other proteins.
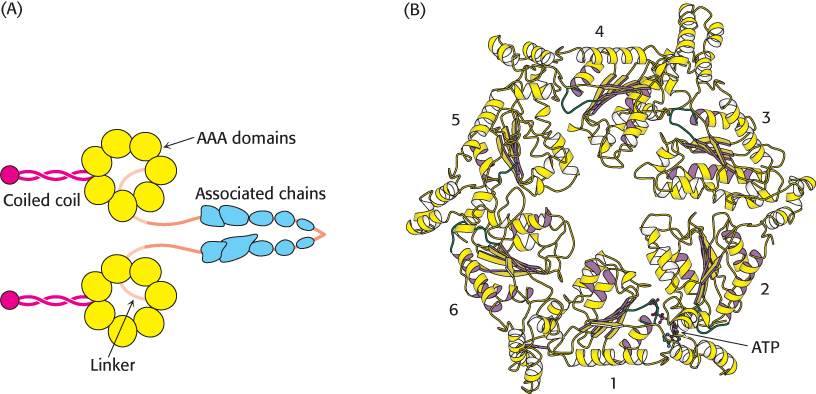
 FIGURE 35.8 Dynein structure. (A) The overall structure of dynein. (B) A model of the motor domain of dynein. Notice the six P-loop NTPase domains, some of which bind to and hydrolyze ATP.
FIGURE 35.8 Dynein structure. (A) The overall structure of dynein. (B) A model of the motor domain of dynein. Notice the six P-loop NTPase domains, some of which bind to and hydrolyze ATP.
[Drawn from 1HN5.pdb.]
Although the structures of these three classes of molecular motors have significant differences, some common features emerge. Almost all structures are dimeric with two head domains, have regions of extended but quite rigid structures, and have regions for interacting with other proteins. As we shall see, these structures are suitable for actions that resemble climbing a rope, hand over hand. The regions for interacting with other proteins represent the grasping hands, the extended structures represent the arms that act as levers to promote larger-scale motion, and the head domains are the engines that provide the necessary mechanical energy.
ATP binding and hydrolysis induce changes in the conformation and binding affinity of motor proteins
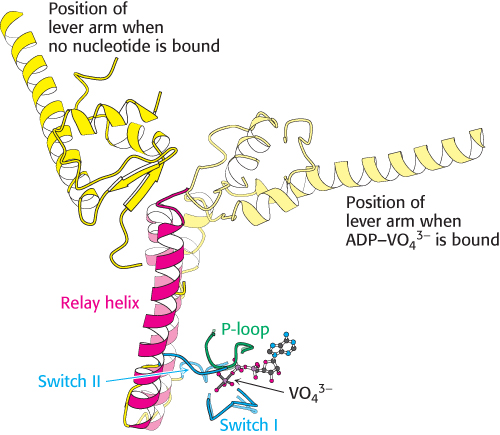
FIGURE 35.10Relay helix. A superposition of key elements in two forms of scallop myosin reveals the structural changes that are transmitted by the relay helix from the switch I and switch II loops to the base of the lever arm. Notice that the switch I and switch II loops interact with VO43− in the position that would be occupied by the γ-phosphoryl group of ATP. The structure of the myosin–ADP–VO43− complex is shown in lighter colors.
[Drawn from 1DFL.pdb and 1SR6.pdb.]
In Chapter 9, we examined the conformational changes that take place in the myosin ATPase domain from the slime mold Dictyostelium. The structures of myosin ATP domains from other sources have been elucidated in a variety of forms as well. The S1 fragment of myosin from scallop muscle provides a striking example of the changes observed (Figure 35.9). The structure of this S1 fragment has been determined in a number of forms including that without bound nucleotide and that bound to a complex formed of ADP and vanadate (VO43−), which, as mentioned in Chapter 9, is an analog of the ATP-hydrolysis transition state. The long helix that binds the light chains (hereafter referred to as the lever arm) protrudes outward from the head domain. Comparison of the structures reveals that the lever arm has rotated by nearly 90 degrees in the ADP–VO43− complex compared with its position in the nucleotide-free form. How does the species in the nucleotide-binding site cause this dramatic transition? Two regions around the nucleotide-binding site (termed switch I and switch II) tightly conform to the shape of the γ-phosphoryl group of the ATP analog and adopt a looser conformation when the γ-phosphoryl group is absent (Figure 35.10). This conformational change allows a long α helix (termed the relay helix) to adjust its position. The carboxyl-terminal end of the relay helix interacts with structures at the base of the lever arm, and so a change in the position of the relay helix leads to a reorientation of the lever arm.
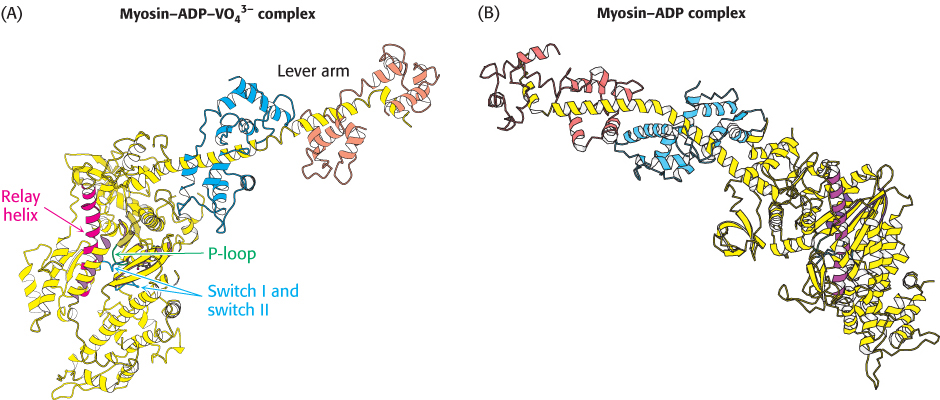
 FIGURE 35.9 Lever-arm motion. Two forms of the S1 fragment of scallop-muscle myosin. Notice the dramatic conformational changes when the identity of the bound nucleotide changes from the ADP–VO43− complex (A) to the nucleotide-free form (B) or vice versa, including a nearly 90-degree reorientation of the lever arm.
FIGURE 35.9 Lever-arm motion. Two forms of the S1 fragment of scallop-muscle myosin. Notice the dramatic conformational changes when the identity of the bound nucleotide changes from the ADP–VO43− complex (A) to the nucleotide-free form (B) or vice versa, including a nearly 90-degree reorientation of the lever arm.
[Drawn from 1DFL.pdb and 1SR6.pdb.]
Analogous conformational changes take place in kinesin. The kinesins also have a relay helix that can adopt different configurations when kinesin binds different nucleotides. Kinesin lacks an α-helical lever arm, however. Instead, a relatively short segment termed the neck linker changes conformation in response to nucleotide binding (Figure 35.11). The neck linker binds to the head domain of kinesin when ATP is bound but is released when the nucleotide-binding site is vacant or occupied by ADP.
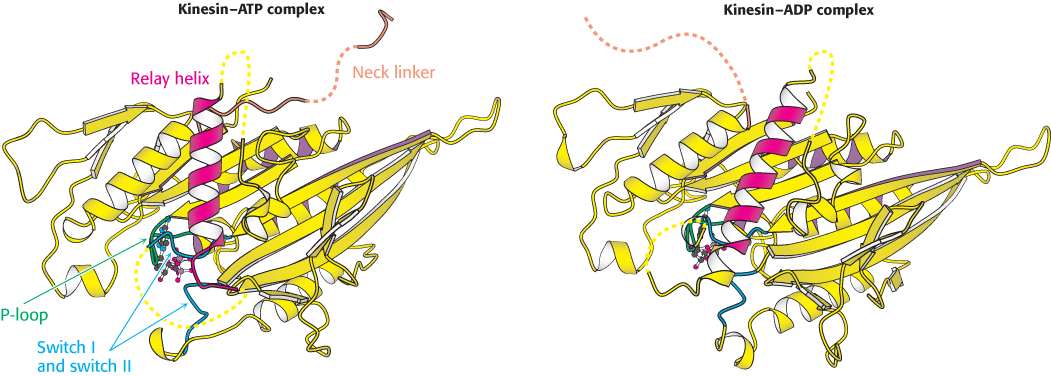
 FIGURE 35.11 Neck linker. A comparison of the structures of a kinesin bound to ADP and bound to an ATP analog. Notice that the neck linker (orange), which connects the head domain to the remainder of the kinesin molecule, is bound to the head domain in the presence of the ATP analog but is free in the presence of ADP only.
FIGURE 35.11 Neck linker. A comparison of the structures of a kinesin bound to ADP and bound to an ATP analog. Notice that the neck linker (orange), which connects the head domain to the remainder of the kinesin molecule, is bound to the head domain in the presence of the ATP analog but is free in the presence of ADP only.
[Drawn from 1I6I.pdb and 1I5S.pdb.]


 FIGURE 35.3 Myosin structure at high resolution. The structure of the S1 fragment from muscle myosin reveals the presence of a P-
FIGURE 35.3 Myosin structure at high resolution. The structure of the S1 fragment from muscle myosin reveals the presence of a P-
 FIGURE 35.5 Myosin two-
FIGURE 35.5 Myosin two-
 FIGURE 35.4 Myosin light chains. The structures of the essential and regulatory light chains of muscle myosin are compared with the structure of calmodulin. Notice the similarities in the structures that allow each of these homologous proteins to bind an α helix (not shown) by wrapping around it.
FIGURE 35.4 Myosin light chains. The structures of the essential and regulatory light chains of muscle myosin are compared with the structure of calmodulin. Notice the similarities in the structures that allow each of these homologous proteins to bind an α helix (not shown) by wrapping around it.

 FIGURE 35.7 Structure of head domain of kinesin at high resolution. Notice that the head domain of kinesin has the structure of a P-
FIGURE 35.7 Structure of head domain of kinesin at high resolution. Notice that the head domain of kinesin has the structure of a P-

 FIGURE 35.8 Dynein structure. (A) The overall structure of dynein. (B) A model of the motor domain of dynein. Notice the six P-
FIGURE 35.8 Dynein structure. (A) The overall structure of dynein. (B) A model of the motor domain of dynein. Notice the six P-

 FIGURE 35.9 Lever-
FIGURE 35.9 Lever-
 FIGURE 35.11 Neck linker. A comparison of the structures of a kinesin bound to ADP and bound to an ATP analog. Notice that the neck linker (orange), which connects the head domain to the remainder of the kinesin molecule, is bound to the head domain in the presence of the ATP analog but is free in the presence of ADP only.
FIGURE 35.11 Neck linker. A comparison of the structures of a kinesin bound to ADP and bound to an ATP analog. Notice that the neck linker (orange), which connects the head domain to the remainder of the kinesin molecule, is bound to the head domain in the presence of the ATP analog but is free in the presence of ADP only.