3.3 WEAK CHEMICAL INTERACTIONS
The macromolecules of most interest to molecular biologists—
When arranged in ordered groups, weak bonds can persist for a long time and can thus play central roles in the formation and stability of the active three-
Three kinds of weak chemical interactions are important in biological systems: van der Waals forces, hydrophobic interactions, and hydrogen bonds. Most macromolecules also use weak ionic interactions, along with these weak chemical interactions, to bind other molecules and to form three-
74
Van der Waals Forces Are Nonspecific Contacts between Atoms
The Dutch chemist Johannes van der Waals was the first to document the intermolecular forces that result from the polarization of atoms. When two atoms approach each other, as they get closer, induced fluctuating charges between them cause a weak, nonspecific attractive interaction. This van der Waals interaction depends heavily on the distance between the interacting atoms. As the distance decreases below a certain point, a more powerful van der Waals repulsive force is caused by overlap of the atoms’ outer electron shells. The van der Waals radius of an atom is defined as the distance at which these attractive and repulsive forces are balanced and is characteristic for each atom (Figure 3-16).
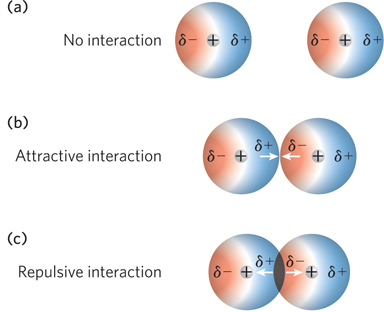
The van der Waals bonding energy between two atoms that are separated by the sum of their van der Waals radii increases with the size of the atoms, but on average is only about 1 kcal/mol—
The strongest kind of van der Waals contact arises when a macromolecule contains a surface that precisely fits the shape of the molecule that it binds. This is the case for antibodies, proteins that recognize antigens—
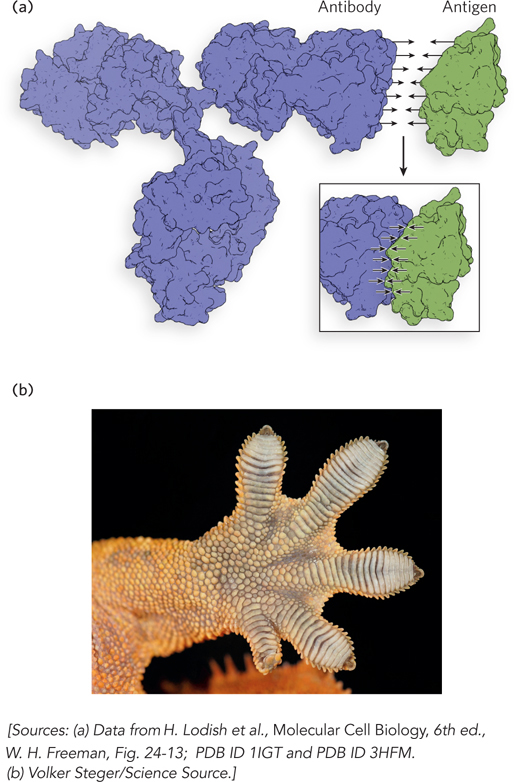
The Hydrophobic Effect Brings Together Nonpolar Molecules
The hydrophobic effect arises from the strong tendency of water to exclude nonpolar groups, forcing these groups to aggregate in contact with one another. The word hydrophobic means “water-
75
Adjacent Bases in Nucleic Acids Participate in Noncovalent Interactions
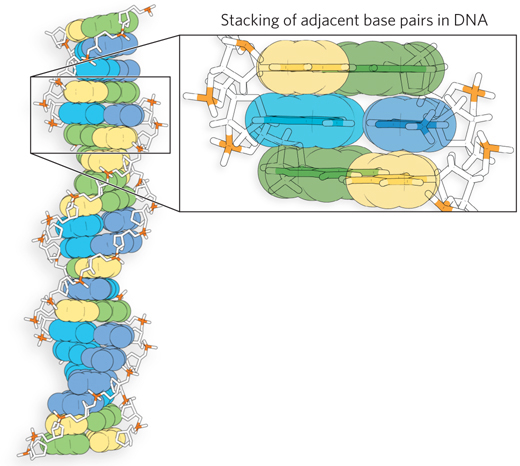
The helical structures in DNA and RNA are stabilized by electronic interactions that arise from the stacked arrangement of base pairs. In accordance with the molecular orbital model introduced earlier, the electrons of the atoms in a base’s aromatic ring(s) are found in a decentralized cloud above and below the ring(s). Pi stacking is defined as the attractive, noncovalent interactions resulting from overlap between electrons of neighboring aromatic rings. This overlap of pi electrons of adjacent stacked nucleotides contributes to the stability of the nucleic acid’s helical structure (Figure 3-18).
Hydrogen Bonds Are a Special Kind of Noncovalent Bond
A hydrogen bond is an attractive intermolecular force between two partial electrical charges of opposite polarity. As the name implies, one partner in the bond is a hydrogen, which must be covalently bonded to a strongly electronegative atom such as oxygen, nitrogen, or fluorine; this hydrogen is the hydrogen-
76
The hydrogen bond is not like a simple attraction between positive and negative charges at two points in space, but instead has directional preference and some characteristics of a covalent bond. This covalent character is more pronounced when acceptors hydrogen-
The chemical properties of water are mostly due to hydrogen bonds that cause water molecules to have strong attractions for one another, but hydrogen bonds can and do form among many other kinds of molecules as well. In proteins, hydrogen bonds occur between the backbone carbonyl and imino groups to stabilize α helices and β sheets, the basic motifs of protein structure that we discuss in Chapter 4. In nucleic acids, hydrogen bonding between complementary pairs of nucleotide bases links one DNA strand to its partner, forming the double helix (see Figure 6-11).
Combined Effects of Weak Chemical Interactions Stabilize Macromolecular Structures
The noncovalent interactions we have discussed (van der Waals forces, hydrophobic effects, pi bonds/stacking, and hydrogen bonds) are substantially weaker than covalent bonds. Just 1 kcal is needed to disrupt a mole of typical van der Waals interactions, but nearly 100 times more energy is required to break an equivalent number of covalent C–
As mentioned earlier, although these types of interactions are individually weak relative to covalent bonds, the cumulative effect of many such interactions can be significant. Macromolecules, including DNA, RNA, and proteins, contain so many sites of possible van der Waals or hydrophobic contacts and hydrogen bonding that the combined effect of these small binding forces is largely responsible for their molecular structure. For macromolecules, the most stable structure is usually that in which weak interactions are maximized. This principle determines the folding of a single polypeptide or polynucleotide chain into its three-
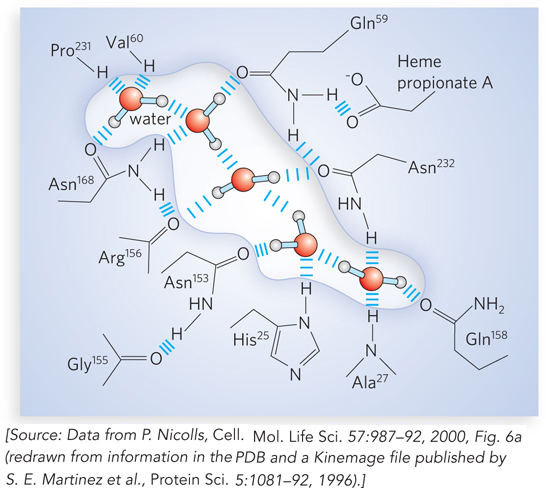
A special class of weak interactions in macromolecules involves the water molecules that are invariably bound to surface and interior sites by hydrogen bonds. Sometimes these water molecules are so well positioned that they behave as though they are an integral part of the macromolecule. In many cases, bound water molecules are essential to macromolecular function. Certain DNA-
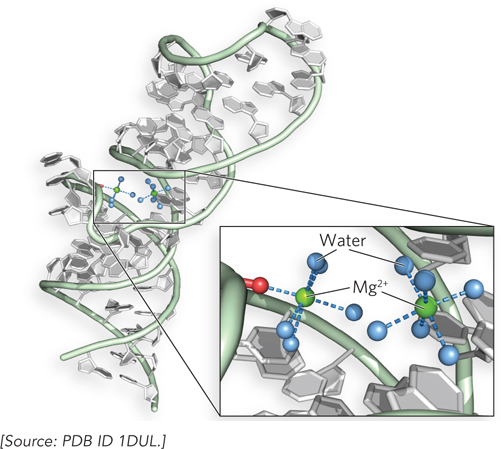
77
Weak Chemical Bonds Also Facilitate Macromolecular Interactions
Interactions among DNA, RNA, and protein molecules, and interactions between these macromolecules and small organic molecules, are also mediated by weak, noncovalent chemical interactions. Such contacts involving information-
For example, the noncovalent binding of a protein to another protein, to a small molecule (such as a hormone), or to another macromolecule (such as a nucleic acid) may involve several hydrogen bonds and one or more ionic, hydrophobic, and/or van der Waals interactions (Figure 3-21). These weak contacts contribute, overall, to an energetically favorable interaction. It is worth noting, however, that because each hydrogen bond between two groups in a protein or nucleic acid forms at the expense of two hydrogen bonds between the same groups and two water molecules, the net stabilization is not as great as it might seem. The large size of nucleic acids and proteins provides extensive molecular surfaces with many opportunities for weak interactions with other molecules. The energetic favorability of such interactions reflects the molecular surface complementarity and the resulting large numbers of weak interactions of polar, charged, and hydrophobic groups on the surfaces of these molecules.
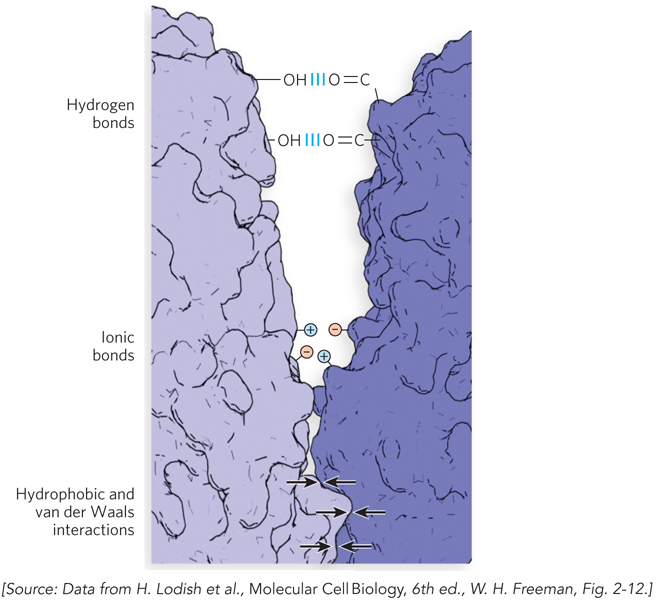
SECTION 3.3 SUMMARY
Weak chemical bonds differ from covalent or ionic bonds in several ways: they involve greater distances between atoms, they are easily broken, and they are often transient. These properties are useful when short-
lived chemical interactions are required in cells. Weak bonds often mediate the interactions of proteins with small molecules, DNA, hormones, or other proteins. Van der Waals forces, hydrophobic effects, and hydrogen bonds are the three most important kinds of weak chemical interactions found in macromolecules that facilitate binding to other molecules and the formation of three-
dimensional structures. Weak ionic interactions are also used. Van der Waals forces occur when two atoms closely approach each other, inducing fluctuating charges that cause a weak, nonspecific, attractive interaction between them. Each type of atom has its own van der Waals radius, the distance at which attractive and repulsive forces with neighboring atoms are balanced.
The hydrophobic effect, giving rise to “hydrophobic interactions,” arises from the strong tendency of water to exclude nonpolar groups, forcing these groups into contact with one another. Hydrophobic contacts stabilize protein structures and the stacking of bases in DNA and RNA helices.
Overlap of pi electrons of adjacent stacked nucleotides contributes to the stability of the helical structure of nucleic acids.
Hydrogen bonds occur between two atoms with partial electrical charges of opposite polarity, one of which is a hydrogen atom. Other types of atoms can also acquire partial positive charge, but only hydrogen atoms are small enough to approach another atom or molecule close enough for an energetically useful interaction.
Although weak chemical interactions have only minor attractive or repulsive effects individually, the cumulative effects can be significant. DNA, RNA, and proteins contain so many sites of possible van der Waals or hydrophobic contacts and hydrogen bonding that the combined effect of many small binding forces is largely responsible for their molecular structure.
78