13.3 HOMOLOGOUS RECOMBINATION IN EUKARYOTES
The repair of double-strand breaks that occur during replication is a key function of recombination systems in all cells. However, with the evolution of more complex cells and organisms, recombination systems underwent adaptation to carry out additional functions related to maintenance of the genome and its transmission from one generation to the next. One well-studied example is the recombination that accompanies the process of meiosis. The diploid germ-line precursor cell has two complete sets of chromosomes, or two genome equivalents. The two copies of each eukaryotic chromosome—the homologous chromosomes, or homologs—generally have the same genes distributed along their lengths, in the same order. However, the genes of the two homologs are not identical. The alleles inherited from each parent are often slightly different, distinguished by mutations and even by small insertions and/or deletions.
During meiosis, each pair of homologs is replicated to create four chromosome copies—two copies, or chromatids, per homolog (see Chapter 2 for a review of meiosis and the stages of the cell cycle). Homologous sister chromatid pairs are aligned during the first meiotic prophase, prophase I. The four sets of chromosomes are then segregated through two cell divisions, meiosis I and meiosis II, to create four haploid gametes (or spores, in lower eukaryotes such as fungi), each with a complete (haploid) chromosomal complement (Figure 13-16). Homologs segregate in the first cell division, and sister chromatids segregate in the second.
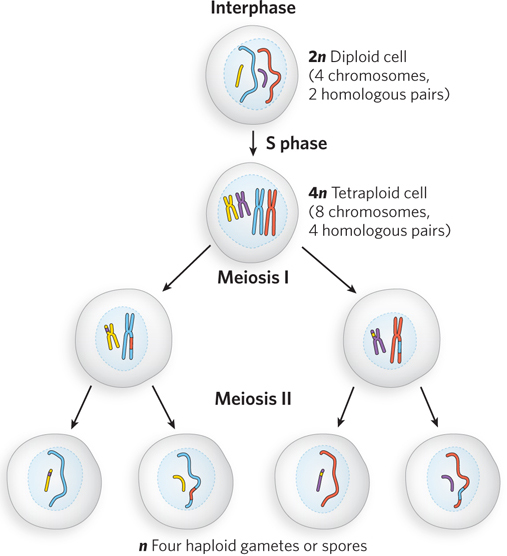
Figure 13-16: An outline of eukaryotic meiosis. DNA is replicated in S phase. For the two pairs of chromosomes shown here, this generates four sets of sister chromatids. The homologs are aligned in meiosis I, joined by recombination (crossovers not shown), then segregated. In meiosis II, the sister chromatids segregate into the haploid cell products. (See also Figure 2-12, right.)
The proper segregation of chromosomes when eukaryotic cells divide requires the attachment of spindle fibers to the centromeres. Chromosomes are then drawn toward opposite poles into what will eventually become two new cells. If replicated chromosomes were scattered randomly about, and spindle fibers were attached randomly to the chromosomes, segregation of the chromosomes to daughter cells would be equally random. A given cell might receive two copies of some chromosomes and no copy of others. To segregate a complete set of chromosomes to each cell, some organizational accounting is required. Chromosome pairs are lined up and linked together in meiosis I. When spindles at opposite poles attach to the centromeres of a linked pair of chromosomes and start to pull, tension is created. This tension, sensed by a mechanism not yet understood, indicates that this pair of chromosomes is properly aligned for segregation. Once the tension is sensed, the links between the chromosomes are gradually removed so that segregation can proceed. If an improper attachment of spindle fibers occurs (e.g., if the two centromeres in the chromosome pair are linked to spindle fibers emanating from the same pole), a cellular kinase senses the lack of tension and activates a system that removes the spindle attachments, allowing the cell to try again.
To generate the needed tension, a physical link between chromosomes destined to be segregated is a requirement for any orderly cell division. During mitosis, and in meiosis II, the sister chromatids produced by replication are segregated. The physical link is provided by cohesins (see Figure 9-25). Cohesins are deposited along a chromosome as it is replicated, ensuring that only homologous sister chromatids are linked together. These cohesin-based links ensure accurate chromosomal segregation by the spindle fibers. However, the segregation of homologs during meiosis I is an event unique to meiosis. The homologs to be segregated are not related by a recent replication event in which cohesins were deposited, and thus some other molecular device is needed to ensure that only homologous chromosomes are linked. The eukaryotic answer to this problem is homologous recombination, a process that relies on closely related sequences in the two chromosomes. Recombinational crossovers provide for the accurate alignment of homologs at the metaphase plate during meiosis I (Highlight 13-2). Many of the same enzymes are involved in both recombinational DNA repair during replication and meiotic recombination. The process again begins with DSBs, but in meiosis I, these breaks do not happen by chance. Instead, they are programmed events.
HIGHLIGHT 13-2 MEDICINE: Why Proper Chromosomal Segregation Matters
When chromosomal alignment and recombination are not correct and complete in meiosis I, segregation of chromosomes can go awry. Aneuploidy, a condition in which a cell has the wrong number of chromosomes, can result. The haploid products of meiosis (gametes or spores) may have no copies or two copies of a chromosome. When a gamete with two copies of a chromosome undergoes fertilization, cells in the resulting embryo are trisomic for (have three copies of) that chromosome.
In S. cerevisiae, aneuploidy resulting from errors in meiosis occurs at a rate of about 1 in 10,000 meiotic events. In fruit flies, the rate is about one in a few thousand (see Figure 2-15). Rates of aneuploidy in mammals are considerably higher. In mice, the rate is 1 in 100, and it is even higher in other mammals. The rate of aneuploidy in fertilized human eggs has been estimated as 10% to 30%, mostly with monosomies or trisomies. This is almost certainly an underestimate. Most trisomies are lethal, and many result in abortive miscarriage long before the pregnancy is detected. This is the leading cause of pregnancy loss. The few trisomic fetuses that survive to birth generally have three copies of chromosomes 13, 18, or 21 (trisomy 21 is Down syndrome). Abnormal complements of the sex chromosomes are also found in the human population. Almost all monosomies are fatal in the early stages of fetal development. The societal consequences of aneuploidy in humans are considerable. Aneuploidy is the leading genetic cause of developmental and mental disabilities. At the heart of these high rates is a feature of meiosis in female mammals that has special significance for the human species.
In a human male, germ-line cells begin to undergo meiosis at puberty, and each meiotic event requires a relatively short period of time. In contrast, meiosis in the germ-line cells of human females is a highly protracted process. The production of an egg begins with the onset of meiosis in the fetus, at 12 to 13 weeks of gestation. This initiation of meiosis occurs in all the developing fetal germ-line cells over a period of a few weeks. The cells proceed through much of meiosis I. Chromosomes line up and generate crossovers, continuing just beyond the pachytene phase (see Figure 13-17)—and then the process stops. The chromosomes enter an arrested phase called the dictyate stage, with the crossovers in place, a kind of suspended animation where they remain as the female matures—for anywhere between 13 and 50 years. It is not until sexual maturity that individual germ-line cells continue through the two meiotic cell divisions to produce egg cells.
Between the onset of the dictyate stage and the final completion of meiosis, something can happen that disrupts or damages the crossovers linking homologous chromosomes in the germ-line cells. As a woman ages, the rate of trisomy in the egg cells she produces increases, dramatically so as she approaches menopause (Figure 1). There are many hypotheses on why this occurs, and several different factors may play a role. However, most of the hypotheses are centered on recombination crossovers in meiosis I and their stability over the protracted dictyate stage.
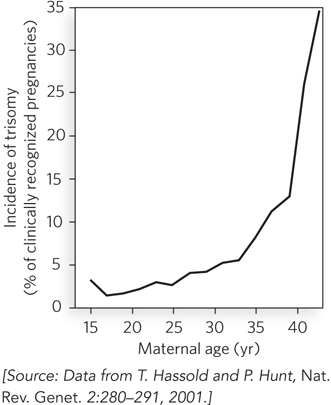
FIGURE 1 The increasing incidence of trisomy with increasing age of the mother.
It is not yet clear what medical steps could be taken to reduce the incidence of aneuploidy in females of child-bearing age. What is revealed is the inherent importance of recombination and crossover generation in human meiosis.
As we discussed in Section 13.1, eukaryotic recombination is not restricted to meiosis. Less frequent recombination events occur during mitosis, at least some of which are associated with the repair of stalled or collapsed replication forks. In addition, recombination systems have been appropriated during evolution to carry out some highly directed exchanges of genetic information between different segments of chromosomes, as we discuss later in this section.
Meiotic Recombination Is Initiated at Double-Strand Breaks
Following the premeiotic S-phase replication cycle, homologous chromosomes are brought together. As the cell enters meiosis, in early prophase I, DSBs are introduced at multiple locations along one chromatid of each chromatid pair (Figure 13-17). The breaks are not random, and yet are not entirely predictable. Certain chromosomal sites, often referred to as hot spots, are much more likely to undergo a break than others. The hot spots are defined by features of chromosome structure not yet fully characterized. An open chromatin configuration, active transcription, and G≡C-rich sequences can all affect the process. The DSBs lead first to processing of the broken ends to generate 3′ extensions and then to strand invasion, as outlined in Figure 13-2. Some of these strand invasions are eliminated, but some undergo additional reactions to become stable crossovers, linking the two pairs of chromatids, as described below. The strand invasions are generally completed early in prophase I, during the leptotene subphase. They are processed into double Holliday intermediates and, finally, to stable crossovers during the succeeding zygotene and pachytene subphases (see Figure 13-17).
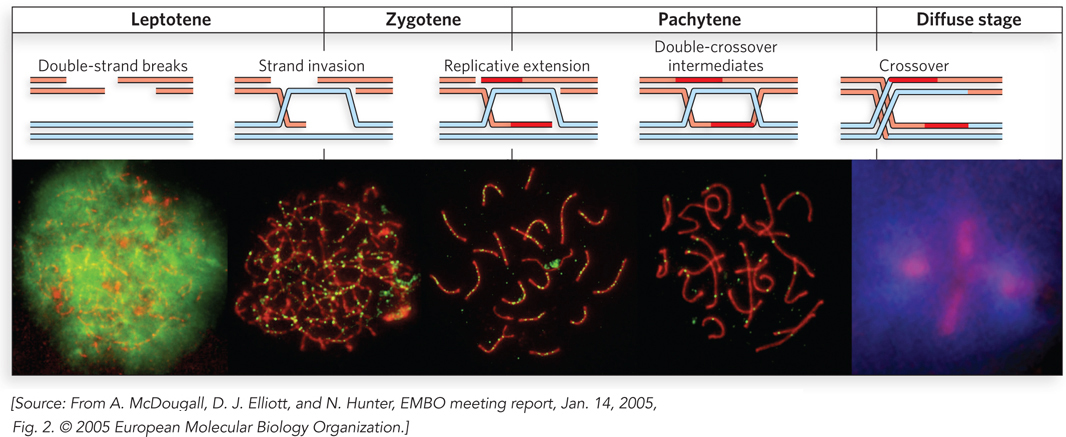
Figure 13-17: Homologous genetic recombination during meiotic prophase I. Prophase I includes a directed recombination process. The double-strand breaks are created and processed early. Strand invasions and replicative extension produce double-crossover intermediates, and some of these mature into stable crossovers. Shown here are the early stages of meiosis in mouse spermatocytes. (Leptotene, zygotene, and pachytene are terms used to describe the subphases of meiosis prophase I.) The progress of the recombination reactions is shown in the upper panel, matched to the subphases of prophase in the microscopic images in the lower panel. The chromosomes are viewed by staining particular proteins through immunofluorescence. Chromosomes are stained red, and a few recombination proteins, fused to green fluorescent protein, appear as green foci. As prophase progresses, the homologous chromosome pairs, at first diffuse, become tightly aligned, producing rodlike structures. The chromosomes again become diffuse as prophase ends and the cells begin the first cell division.
The enzymes that carry out this process are best characterized in the yeast Saccharomyces cerevisiae, although similar enzymes exist in all eukaryotic cells. A protein called Spo11 (so named because inactivation of this protein causes defects in yeast sporulation), closely related to eukaryotic type II topoisomerases, catalyzes formation of DSBs (Figure 13-18a; see the How We Know section at the end of this chapter). Spo11 is found in all eukaryotes. Acting as a dimer, it uses an active-site Tyr residue as a nucleophile in a transesterification reaction (Figure 13-18b). Each subunit cleaves one DNA strand, with the phosphodiester bond replaced by a 5′-phosphotyrosyl linkage. The reaction halts at this point, and Spo11 does not carry out the additional steps of a topoisomerase reaction. A dozen or more additional proteins may cooperate in the formation of an active Spo11 complex on the DNA and in processing the DNA after it is cleaved.
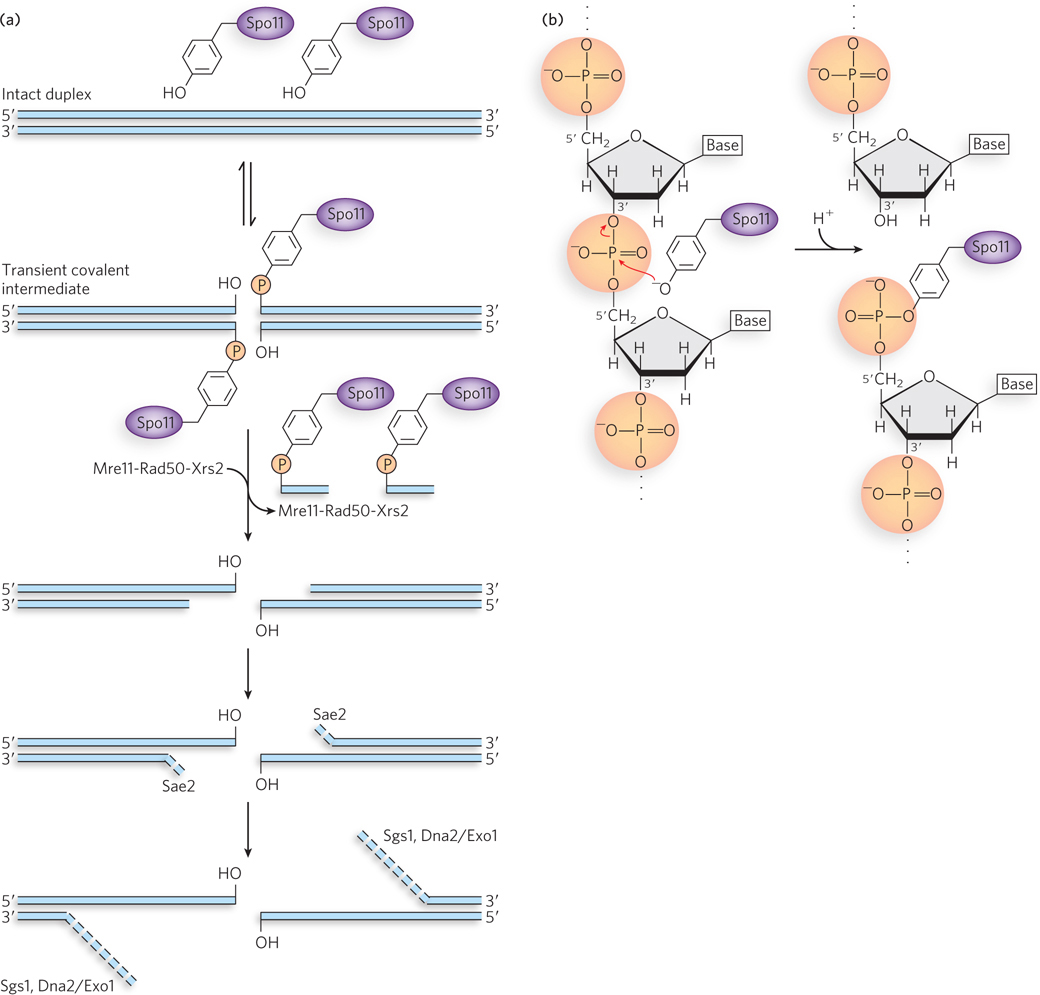
Figure 13-18: The Spo11 protein and initiation of meiotic recombination. (a) The reaction promoted by the Spo11 protein and subsequent processing of the double-strand break. An active-site Tyr residue acts as nucleophile, attacking the phosphodiester bond. The 3′ hydroxyl is displaced, creating a 5′ phosphotyrosyl-DNA complex. Once the DSB is formed, the broken ends are processed by the action of the Mre11-Rad50-Xrs2 complexes, Sae2 endonuclease, Sgs1 helicase, and ExoI exonuclease or Dna2, which has both helicase and 5′→3′ exonuclease activities. Rad51 or Dmc1 is loaded onto the 3′ extensions (not shown), and strand invasion proceeds. (b) Details of the Spo11 cleavage (transesterification) reaction.
To remove Spo11 from the DNA and initiate nucleolytic degradation of the 5′-ending strand, a complex of proteins consisting of Mre11 (meiotic recombination), Rad50 (radiation sensitive), and Xrs2 (x-ray sensitive) binds to each Spo11 complex and cleaves the DNA on the 3′ side of Spo11, liberating the linked protein along with a short segment of the attached 5′-ending DNA strand. The nuclease Sae2 degrades the same DNA strand a bit more. Three other enzymes, the helicase Sgs1 and the nucleases Dna2 or Exo1, have been implicated in additional degradation of the 5′ ends to create long 3′ single-stranded extensions (see this chapter’s Moment of Discovery for a description of experiments leading to discovery of the role of Sgs1 in this process). The single-stranded regions are bound by RPA, the eukaryotic single-stranded DNA–binding protein. Aided by mediator proteins, two RecA-class recombinases, called Dmc1 (disrupted meiotic cDNA) and Rad51, are loaded, perhaps asymmetrically, onto the 3′ extensions on either side of the double-strand break. Dmc1 and Rad51 are the eukaryotic counterparts to RecA. They exhibit sequence and structural homology to the RecA protein and, like RecA, they form extended nucleoprotein filaments on the DNA. The site is now set up for recombination.
Meiotic Recombination Is Completed by a Classic DSBR Pathway
Meiotic recombination is a directed process, which is slowly yielding its secrets to intensive research. The primary goal of recombination is to create physical links, or crossovers, between chromosomes. The formation of crossovers is very tightly regulated so that at least one crossover is created in every pair of homologs. At the same time, a process of interference, not yet understood, ensures that the total number of crossovers for each pair is limited. Where several crossovers occur between the same homologs, they are spaced far apart on the chromosomes. Once the DSBs have formed, subsequent recombination is regulated to occur almost exclusively between homologs rather than between sister chromatids.
The DSBs and Holliday intermediates have two possible fates, only one of which leads to a stable crossover. In one pathway, the invading strand dissociates and pairs with its complement on the other side of the break (Figure 13-19a). Further extension and ligation complete the process. This pathway is equivalent to SDSA. No link between the two chromosomes is created and no crossover occurs. However, even this process is not genetically neutral. Homologous regions may have different alleles of the same gene, with small base-pair differences. If the region where the two single strands are paired contains one of these base-pair differences, the final reannealed duplex will have a mismatch. This is typically repaired by the cellular mismatch repair system to create a normal base pair. The base on one strand or the other must be changed, and genetic information is lost on the changed strand. This type of outcome is referred to as gene conversion. It represents one byproduct of recombination, not only during meiosis but also during the recombinational repair of DSBs and single-strand gaps in other contexts. However, even though a small bit of genetic information may be transferred, the physical crossover has been eliminated.
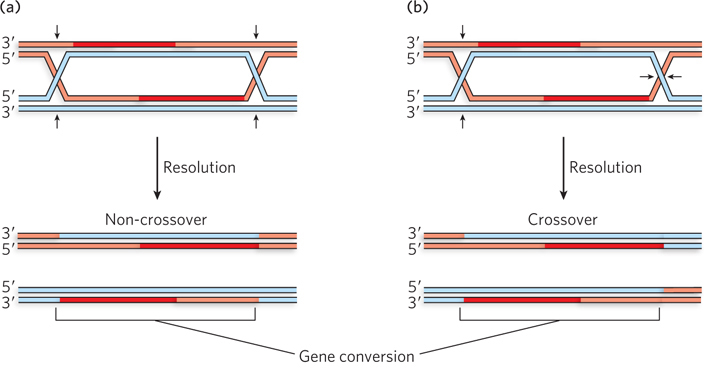
Figure 13-19: The two possible fates of double-strand breaks in meiosis. (a) Recombination with no crossover but some potential for gene conversion. (b) Recombination leading to a genetic crossover. Gene conversion can occur in some regions between the two Holliday intermediates.
In the alternative pathway, strand extension leads to displacement of one strand of the invaded duplex, and this strand eventually pairs with the other side of the DSB (Figure 13-19b). DNA ligation creates a double Holliday intermediate, and thus a physical link between the homologs. As meiosis proceeds, the double Holliday intermediate is cleaved by resolvases (similar to the bacterial RuvC), with the potential to create a stable crossover. The final crossover is embedded in a proteinaceous structure called a chiasma (see Figure 2-18). The overall pathway is closely related to that in a breakthrough model that first described meiotic recombination as a process of DSBR, proposed by Jack Szostak, Terry Orr-Weaver, Rodney Rothstein, and Frank Stahl in 1983.
Crossovers have two roles in meiosis. The first is to create the physical link essential for proper chromosomal segregation. The second role is a genetic one. Following segregation, the sister chromatids (now daughter chromosomes) are no longer identical. One end of at least one of each set of paired chromatids has been exchanged with the homolog—a genetic crossover, generating genetic diversity.
Meiotic Recombination Contributes to Genetic Diversity
Meiosis, then, is not simply a mechanism for reducing a diploid genome to a haploid genome in the eukaryotic gamete (or spore). Meiosis increases the genetic diversity in a population by shuffling the genome in two ways. The first is independent assortment of unlinked genes (Figure 13-20). As a cell goes through meiosis I, homologous chromosomes are segregated to the daughter cells. The chromosomes assort independently. If a particular gamete obtains chromosome 1 as a result of the meiotic cycle, that has no bearing on which version of chromosome 2 also ends up in that gamete. Genes on a given chromosome are linked and are likely to be inherited together, but genes on different chromosomes are unlinked.
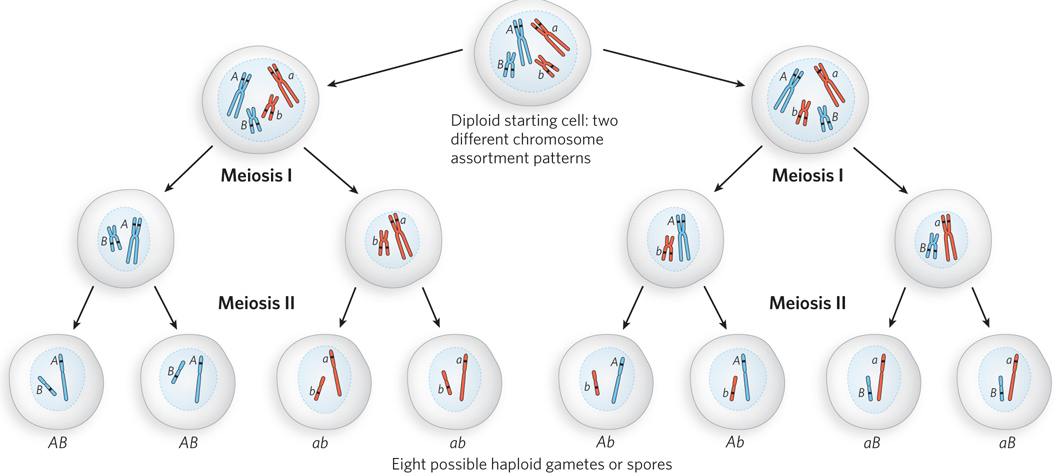
Figure 13-20: The contribution of independent assortment to genetic diversity. Much genetic diversity comes from the independent assortment of chromosomes during meiosis. Blue and red distinguish the chromosomes inherited from each parent. One gene on each chromosome is highlighted, with different alleles (A or a; B or b) in the homologs. Independent assortment can lead to gametes with any combination of alleles on each chromosome.
Recombination during meiosis I makes an additional contribution to the genetic diversity of gametes. Crossovers between homologs in prophase I shuffle the alleles on individual chromosomes. Each gamete ends up with one complete genomic complement, with all genes present. However, given the variation of crossover locations and the unpredictability of independent assortment, the resulting gamete population includes individual cells that may contain virtually any combination of alleles derived from each parent.
Recombination during Mitosis Is Also Initiated at Double-Strand Breaks
Double-strand breaks occur more rarely during mitosis, and recombination is correspondingly less frequent. The breaks can result from endonucleolytic action or exposure to ionizing radiation. However, the most common source of DSBs in mitosis is the encounter of a replication fork with a template strand break (see Figure 13-1). When this occurs, a cellular checkpoint is activated (a signaling pathway) that halts the progression of the cell cycle, and recombinational DNA repair processes are initiated. Mitotic recombination is generally limited to the S and G2 phases of the cell cycle, when sister chromatids are present. Recombination between homologous chromosomes in diploid cells is much less frequent—probably a simple matter of the physical distance between homologs.
The pathways in mitotic recombination are similar to those in meiotic recombination (Figure 13-21). When a DSB is generated by ionizing radiation, it can be repaired by two main pathways: SDSA or DSBR. In both pathways, the break is processed by nucleases to generate 3′ single-stranded tails, which are coated with RPA. With the aid of recombination mediator proteins, the recombinase is loaded onto the single-stranded DNA to promote strand invasion of a double-stranded homologous chromosome, usually the sister chromatid (or, less frequently, the homolog). The Dmc1 recombinase is specific to meiosis, so mitotic recombination relies entirely on Rad51. Recombination mediators include the Rad52 protein in all eukaryotes and, in vertebrates, the BRCA2 protein. These proteins load Rad51 onto RPA-coated single-stranded DNA and may have additional functions. Humans with a defect in the BRCA2 gene or certain defects in the gene for Rad52 have a predisposition to breast cancer and several other cancers. The BRCA1 protein also plays an important, but incompletely defined, role in mediating the overall process. A cellular deficiency in either BRCA1 or BRCA2 results in the accumulation of chromosomes with aberrant structures and aneuploidy. The involvement of these key cancer suppressor genes provides a vivid illustration of the importance of mitotic recombination in maintaining the genome.
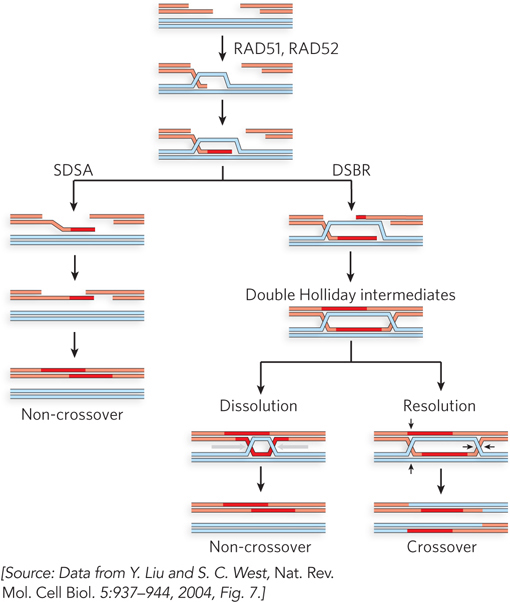
Figure 13-21: Mitotic recombination. The most common path of mitotic recombination is SDSA (left). After strand invasion and strand extension, the invading strand is displaced. It can then anneal with its complement on the other side of the original double-strand break. Replication, nucleolytic trimming as needed, and ligation complete the process. Crossovers occur in recombination events in the DSBR pathway (right). Rad52 protein may be involved in initiation of the second strand invasion that leads to the double Holliday intermediate.
Programmed Gene Conversion Events Can Affect Gene Function and Regulation
Saccharomyces cerevisiae is a single-celled eukaryote, familiar to bakers and brewers and the thousands of scientists who have adopted this yeast as a model organism (see the Model Organisms Appendix). It can live with a haploid genome complement or as a diploid. Both forms are stable and both reproduce by mitosis, with daughter cells budding off mother cells. However, the haploid and diploid forms can also be interconverted under the right conditions. Haploid cells exist in two forms called mating types, designated a and α. A haploid cell can mate with another haploid cell of the opposite mating type (a with α, or α with a), creating a stable diploid. When conditions are unfavorable for growth, diploid cells can undergo meiosis and its accompanying recombination, resulting in four haploid spores (two a spores and two α spores). The spores represent a dormant state that can survive stressful environmental conditions. When conditions improve, the spores can begin growing as haploid cells.
Cells can be converted from one mating type to another, in a process called mating type switching. This provides yet another manifestation of the recombination process. The mating type of a haploid cell is determined by two different versions of a gene expressed at a single locus called MAT. If the MAT locus is MATa, the cell has the a mating type. If the MAT locus is MATα, the cell has the α mating type. The genetic information for MATa and MATα is stored in nearby silent copies of each of these genes, called HMLα and HMRa. That genetic information is transferred to the MAT locus by an unusual mechanism, with recombination at its core (Figure 13-22).
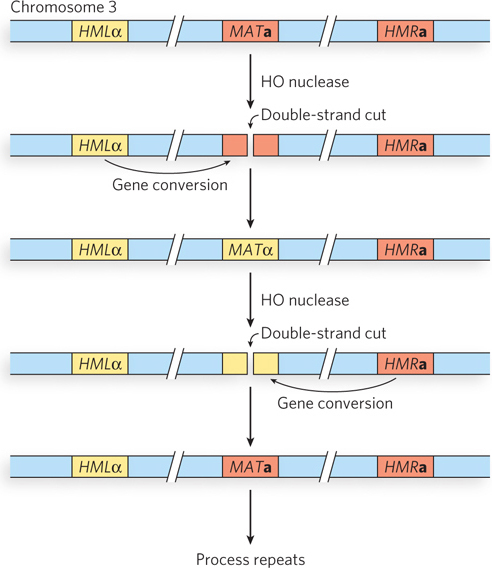
Figure 13-22: The mating-type loci in S. cerevisiae. The genetic information expressed at the MAT locus on chromosome 3 determines mating type (a or α) in yeast. That information can be changed by recombination-mediated gene conversion, moving information (normally silent) that is stored at the HMLα and HMRa loci.
Haploid cells can switch mating types, and they do so as often as every cell generation. The switch requires a directed recombination reaction initiated by a double-strand break. The overall process represents one more example of the steps outlined in Figure 13-2. The MAT locus is cleaved by a nuclease called HO to generate a double-strand break at a specific location near MAT, and recombinational repair of the DSB is directed by recombination with one of two mating-type donor sites (see Figure 13-22). The HMLα locus has 700 bp of α-specific genetic information, and the HMRa locus has 650 bp of a-specific genetic information. (The locus names are derived from homothallic locus left and homothallic locus right.) The genes at HMLα and HMRa are not expressed; these loci serve only as a silent reservoir of genetic information used to change the information at the MAT locus by gene conversion.
The mating-type switch is a classic example of recombinational DNA repair of a double-strand break by the SDSA pathway (Figure 13-23; see also Figure 13-3). If the cell is initially the a mating type, MATa is cleaved by HO, and the free DNA ends are processed to generate 3′ single-stranded overhangs. The Rad51 protein binds to the overhangs and directs DNA strand invasion at a homologous part of the HMLα locus. As the invading 3′ end is extended by a DNA polymerase, the α mating-type information in HMLα is copied. The a mating-type information at the MAT locus is removed by nuclease digestion. Once the extending strand is long enough, it dissociates, the end is paired with a homologous gene segment in the original MAT locus, and the DNA gap is filled in and ligated. MATa is thus switched to MATα. Switching from MATα to MATa is essentially the same reaction, except that the strand invasion occurs at the HMRa locus.
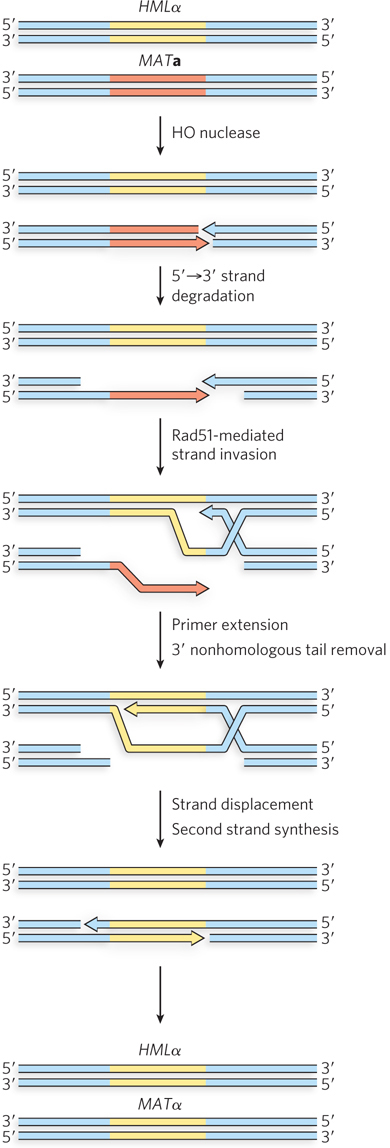
Figure 13-23: Pathway of the mating-type switch in yeast. The double-strand break is introduced by the nuclease HO. The subsequent strand invasion and other steps closely resemble the SDSA pathway. The MATa information is converted to MATα information by the resulting gene conversion.
Similar gene conversion processes arising from the SDSA form of recombination are surprisingly common, found in many bacteria and single-celled eukaryotes. They allow the cell to alternate between two or more states. Examples important to medicine can often be found in pathogens that are able to evade the human immune system. For example, the bacterium Neisseria gonorrhoeae is the agent that causes gonorrhea. The immune response to the pathogen is largely directed at antigens in the bacterial pili, cellular projections involved in adhesion to host cells and other bacteria. The bacterium can evade the host’s immune system by alternately expressing different pilin genes, through antigenic variation. Switching from the expression of one pilin gene to another proceeds by a process very similar to the mating-type switch in yeast. One pilin gene locus is expressed, but the genetic information at that locus can be switched out by genetic recombination with alternate silent gene loci.
The use of recombination to switch expression between different sets of genes has a major advantage over the more common types of gene regulation described in Chapters 19, 20, 21 and 22. It is absolute. The mating-type locus of yeast is either a or α, never partially one or the other. The template donor genes are not expressed at all, because they are not present in the gene expression locus. For a pathogen, even low levels of leaked expression of the wrong genes could undermine its strategy for circumventing the host’s immune system.
Some Introns Move via Homologous Recombination
Some of the introns that are found in many eukaryotic (and a few bacterial) genes have the interesting property that they can move—from one gene to another copy of the same gene on a homologous chromosome that lacks that particular intron. In this way, an intron that becomes associated with a specific gene (say, gene X) can rapidly spread through a population so that all copies of gene X in the population contain that intron. The movements occur whenever genetic transfers bring chromosomes together from two different sources, such as during the fusion of gametes at fertilization.
The movement can occur in several ways, but mobile introns of the group I class (these self-splicing introns are discussed in Chapter 16) use recombinational DNA repair of a targeted double-strand break (Figure 13-24). In brief, the introns encode a homing endonuclease, an enzyme that cleaves a specific sequence in any copy of the host gene that lacks the intron. The resulting DSB is repaired by recombination with the gene that does have a copy of the intron.
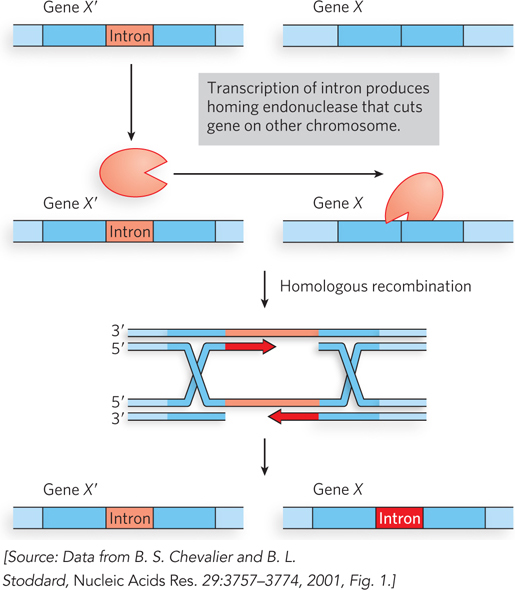
Figure 13-24: The homing pathway of some group I introns. In this pathway, the gene with the intron (X′) and the same gene without the intron (X) are present in the cell, and an intron-encoded endonuclease creates a double-strand break in the uninterrupted gene (X). The break triggers recombinational DSBR similar to that outlined in Figure 13-2, using the intron-containing gene (X′) as a template for repair. When the repair is complete, both genes have a copy of the intron.
Homing endonucleases have been widely used in biotechnology. They function much like restriction enzymes (see Chapter 7), but they recognize and cleave sequences that are much larger, 12 to 40 bp, and are asymmetric. These sites occur very rarely in genomes. If a site recognized by one of these enzymes is engineered into a chromosome or viral DNA, it can be reproducibly cleaved without affecting other genomic sequences.
SECTION 13.3 SUMMARY
During meiosis, recombination generates crossovers that create a physical link between homologous chromosomes just before the first meiotic cell division.
Meiotic recombination events are initiated at programmed double-strand breaks, and most proceed by synthesis-dependent strand annealing.
Meiotic recombination in eukaryotes makes an important contribution to the generation of genetic diversity in a population.
Some meiotic recombination events do not generate crossovers, but instead result in a more subtle exchange of genetic information known as gene conversion.
Mitotic recombination is rarer and is also initiated at DSBs.
Directed homologous recombination promotes a mating-type switch in yeast and can promote antigenic variation in some pathogens.
Some group I introns migrate by means of recombinational DSBR.









