15.1 RNA POLYMERASES AND TRANSCRIPTION BASICS
Transcription in cells and viruses is catalyzed by specialized enzymes called RNA polymerases. The transcription reaction resembles DNA replication in its fundamental chemical mechanism, in the direction of synthesis (5′→3′), and in the requirement for a template strand. And, like replication, transcription has initiation, elongation, and termination phases. In contrast to replication, however, transcription does not require a primer to begin RNA synthesis, and it involves defined sections of DNA rather than the entire molecule. Also, just one of the two strands of a DNA segment serves as the template for a given transcription reaction (Figure 15-1). In this section we discuss the different types of polymerases, steps in the transcription process, and how transcription can be blocked by inhibitors.
RNA Polymerases Differ in Details but Share Many Features
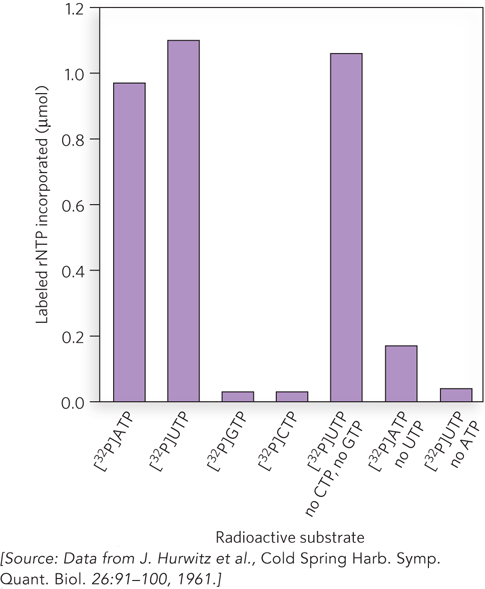
RNA polymerases were first discovered by testing cell extracts for activity that could form an RNA polymer from ribonucleoside 5′-triphosphates (rNTPs). Experiments demonstrated that the RNA product of this polymerase activity was complementary to the sequence of DNA supplied in the reaction mix. A particularly telling experiment involved a DNA strand with an alternating (AT)n sequence. The use of different radiolabeled rNTPs revealed that only ATP and UTP were needed for complete synthesis of RNA, and not GTP or CTP (Figure 15-2). Subsequent experiments using the purified E. coli RNA polymerase, and, later, using bacteriophage RNA polymerases, helped define the fundamental properties of transcription.
521
In addition to a DNA template, DNA-
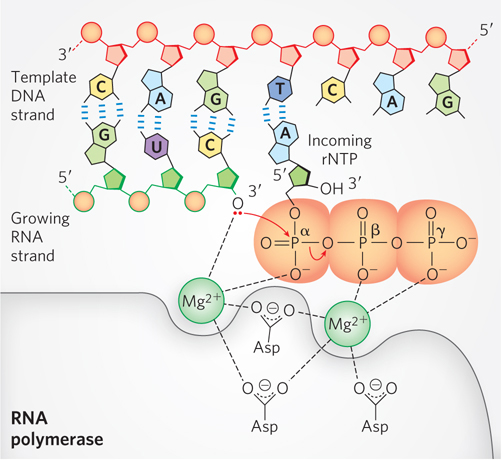
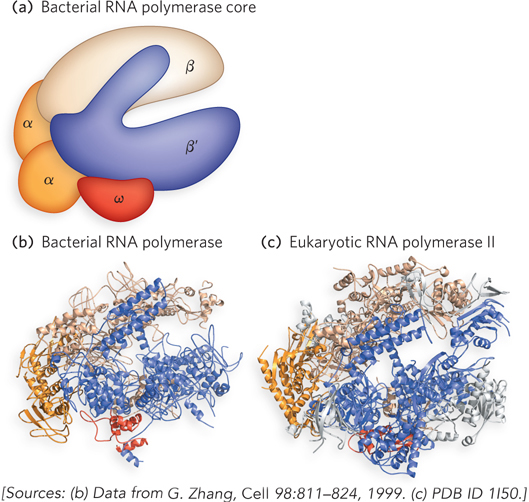
RNA polymerases are fascinating enzymes that continue to be actively studied. The simplest examples consist of one polypeptide chain, such as the phage T7 and Sp6 RNA polymerases. In contrast, all cellular RNA polymerases, from bacteria to humans, are composed of multiple polypeptides that fold together to create the functional enzyme. In E. coli, for example, the RNA polymerase core is a large, complex enzyme with five polypeptide subunits: two copies of the α subunit and one copy each of the β, β′, and ω subunits: α2ββ′ω (Mr 390,000), as shown in Figure 15-4a. A sixth subunit, designated σ and known as sigma factor, binds transiently to the core and directs the enzyme to specific binding sites on the DNA. These six subunits constitute the RNA polymerase holoenzyme. Bacteria have multiple sigma factors, named according to their molecular weight; the most common is σ70 (Mr 70,000). Thus, the RNA polymerase holoenzyme of E. coli exists in several forms, depending on the type of σ subunit it contains. Sigma factors play an important role in the recognition of different types of bacterial genes (see Section 15.2).
522
In eukaryotic cells, three distinct RNA polymerases are responsible for transcribing RNAs with different functions. RNA polymerase I (Pol I) transcribes genes encoding large rRNA precursors. RNA polymerase II (Pol II) transcribes nearly all protein-
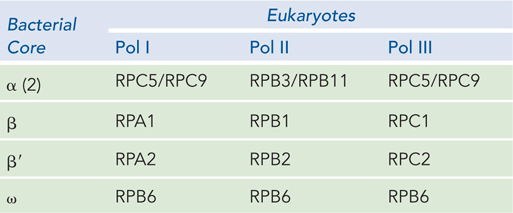
523
The molecular structures of bacterial and yeast RNA polymerases have been determined by x-
Unlike DNA polymerase, RNA polymerase does not require a primer to initiate synthesis. RNA polymerase catalyzes RNA synthesis in three distinct phases, similar to those of the DNA polymerase reaction. Initiation occurs as RNA polymerase binds to specific DNA sequences called promoters. Elongation is the process of adding nucleotides to the growing RNA strand. Termination is the release of the product RNA when the polymerase reaches the end of a gene or other transcription unit.
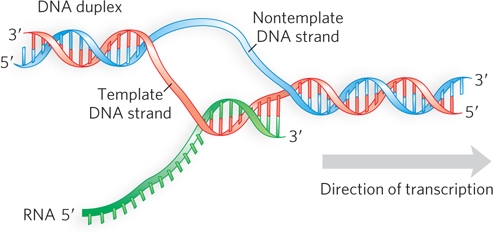
The two strands of a DNA duplex have different roles in transcription. The template strand serves as a template for RNA synthesis, and its complement, the nontemplate strand, is identical in base sequence to the RNA transcribed from the gene, with U in the RNA in place of T in the DNA (Figure 15-5). The nontemplate strand is more often called the coding strand. The coding strand for a particular gene may be located in either strand of a given chromosome.
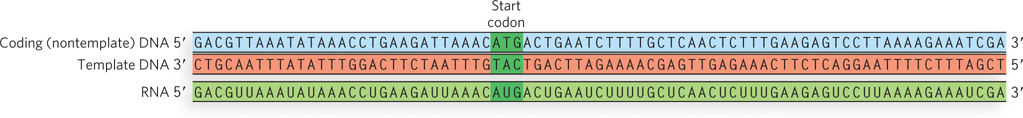
KEY CONVENTION
The template strand of the DNA is copied during transcription, and its sequence is the complement of the RNA transcript. The coding strand of the DNA has the same sequence as the RNA transcript (except for T in the DNA and U in the RNA). Hence, for example, the start codon for transcription—
To enable RNA polymerase to synthesize an RNA strand complementary to the template DNA strand, the DNA duplex must unwind over a short distance, forming what is known as a transcription “bubble” (Figure 15-6). During transcription, the E. coli RNA polymerase generally keeps about 17 bp of DNA unwound. In the elongation phase, the growing end of the new RNA strand base-
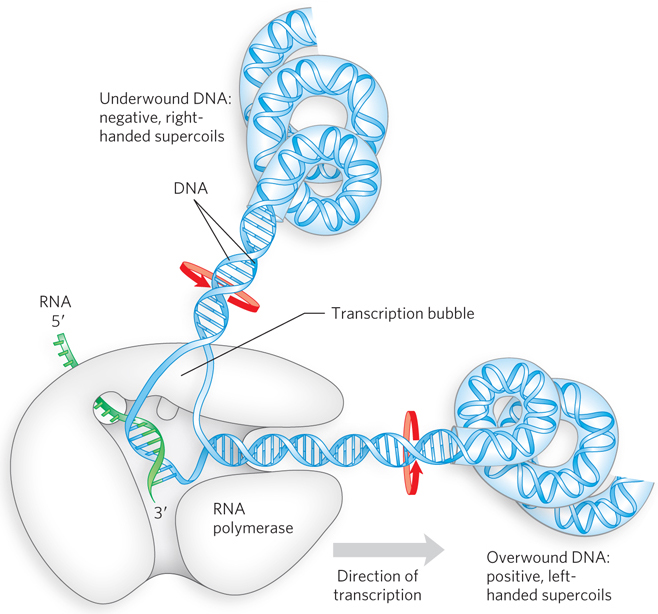
RNA polymerases lack a separate proofreading 3′→5′ exonuclease active site, which exists in many DNA polymerases. Consequently, the error rate for transcription is higher than that for chromosomal DNA replication—
524
Transcription Initiation, Elongation, and Termination Occur in Discrete Steps
The general steps of the transcription pathway are the same in both bacteria and eukaryotes (Figure 15-7). The polymerase binds the promoter (step 1), forming first a closed complex, in which the bound DNA is intact, and then an open complex (step 2), in which the bound DNA is partially unwound near a region 10 bp ahead of (upstream of) the transcription start site. Transcription is initiated within the complex (step 3), leading to a conformational change that converts the complex to the form required for elongation. Promoter clearance, involving movement of the transcription complex down the DNA template and away from the promoter, leads to the formation of a tightly bound elongation complex (step 4). Once elongation begins, RNA polymerase becomes a highly efficient enzyme, completing synthesis of the transcript before dissociating from the DNA template (step 5), then recycling for a new round of transcription. Although the steps in this pathway are conserved across species, the details of the process are somewhat more complex in eukaryotic cells.
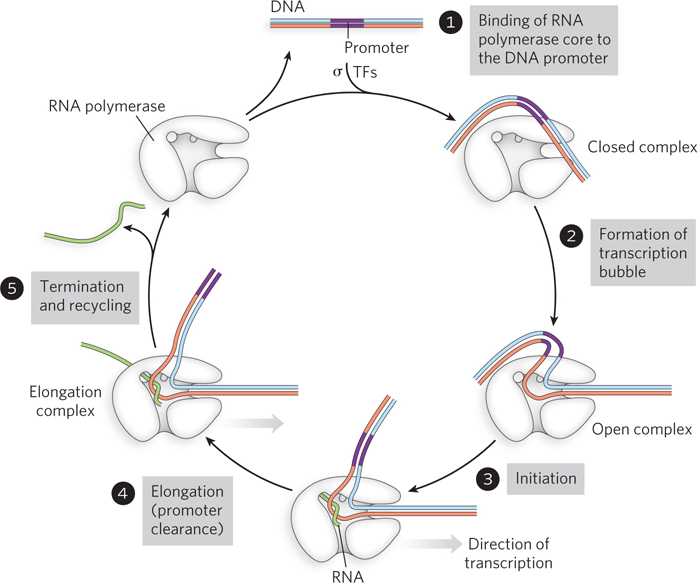
RNA synthesis is processive, which means that once RNA polymerase begins elongating a transcript, the kinetics of the polymerization reaction greatly favor the addition of the next nucleotide over premature release of the transcript. As we will see, elongation is not a uniform process but instead occurs in fits and starts, and specific sequences trigger termination of RNA synthesis by RNA polymerase.
DNA-Dependent RNA Polymerases Can Be Specifically Inhibited
Small molecules and peptides that inhibit transcription are useful both as antibiotics and as tools for research. Actinomycin D, one of a class of peptide antibiotics isolated from Streptomyces soil bacteria, inhibits transcription elongation by RNA polymerase in bacteria and eukaryotes (Figure 15-8). The planar portion of this molecule intercalates into the double-
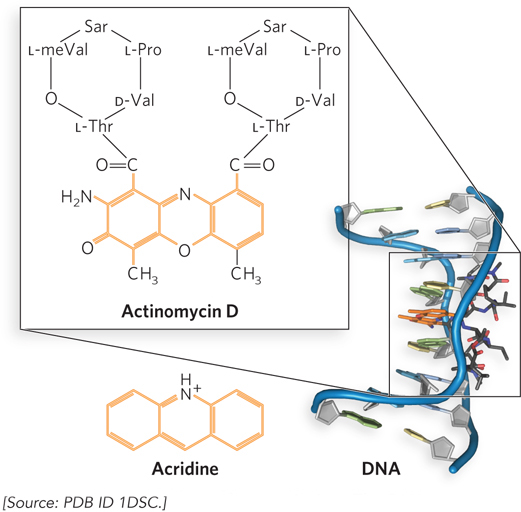
525
Some species rely on transcription inhibitors for natural biodefense. For example, the mushroom Amanita phalloides produces α-amanitin (Figure 15-9a, b), a cyclic peptide that disrupts eukaryotic mRNA synthesis by blocking Pol II and, at higher concentrations, Pol III. The binding position of α-amanitin in Pol II prevents the flexibility required for translocation of the polymerase along the DNA substrate (Figure 15-9c). α-Amanitin is useful in the laboratory as a specific inhibitor of eukaryotic Pol II, or to determine the polymerase responsible for transcribing a particular gene. This can be demonstrated in an experiment monitoring RNA synthesis when an RNA polymerase and rNTPs are combined with a DNA template (Figure 15-9d). Under normal conditions, Pol I produces rRNA, Pol II produces mRNA, and Pol III produces tRNA. The addition of α-amanitin inhibits the synthesis of mRNA, but not that of rRNA or tRNA. Indeed, Pol I, Pol III and bacterial RNA polymerase are insensitive to α-amanitin—
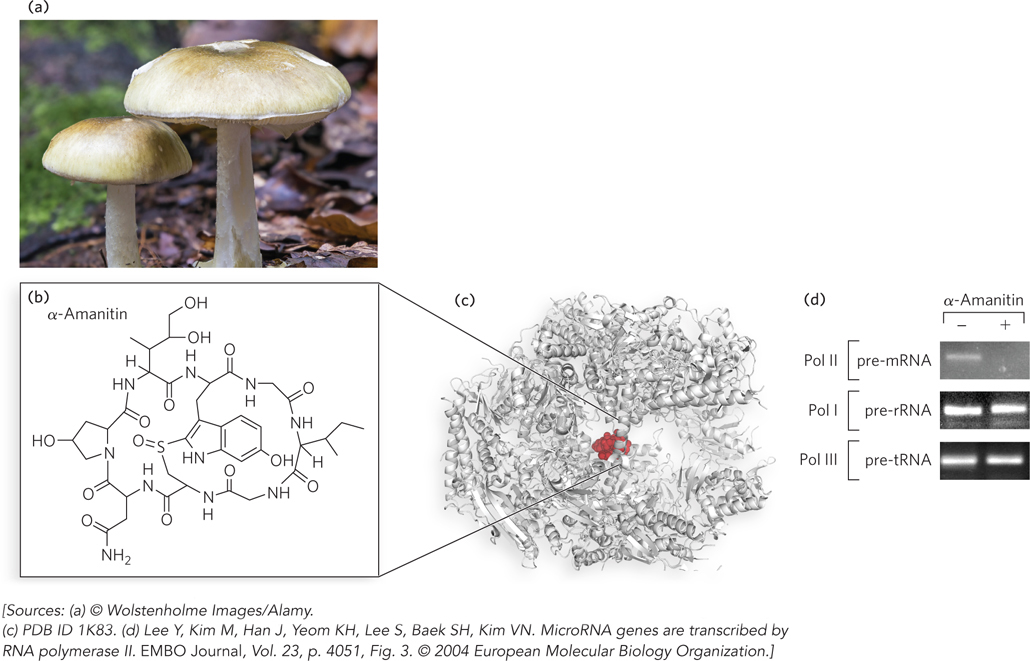
526
Transcriptional Regulation Is a Central Mechanism in the Control of Gene Expression
Transcription is the first step in the complicated and energy-
The DNA sequence in the promoter region affects the efficiency of RNA polymerase binding and the initiation of transcription. However, differences in promoter sequences are just one of several levels of control during initiation. The binding of additional proteins to sequences both near to and distant from the promoter can also affect transcription levels. Protein binding can activate transcription by facilitating RNA polymerase binding or later steps in the initiation process, or it can repress transcription by blocking polymerase activity (activation and repression of transcription by specific proteins are discussed in detail in Chapters 20 and 21).
SECTION 15.1 SUMMARY
Transcription is catalyzed by DNA-
dependent RNA polymerases, which use ribonucleoside 5′-triphosphates to synthesize RNA complementary to the template strand of duplex DNA. The steps of transcription consist of binding of RNA polymerase to a promoter on DNA to form a closed complex, opening of the complex by local DNA unwinding near the promoter, initiation, elongation, and termination. 527
The simplest RNA polymerases, with one polypeptide chain, are found in some bacteriophages. All cellular RNA polymerases are composed of multiple polypeptides that fold together to create the functional enzyme.
Bacterial RNA polymerase uses a sigma (σ) factor to recognize and bind the promoter during initiation.
Eukaryotic cells have three types of RNA polymerases. Pol I and Pol III transcribe genes encoding rRNAs and small functional RNAs such as tRNA, respectively. Pol II transcribes protein-
coding genes to make mRNA. Once an elongation complex forms on a DNA template, RNA polymerase completes the synthesis of the transcript before dissociating from the DNA.
Various naturally occurring small molecules inhibit polymerase enzymes and can be used to detect which polymerase produces specific types of RNA.
Much of the regulation of protein levels in both bacterial and eukaryotic cells occurs during the early stages of transcription.