15.3 TRANSCRIPTION IN EUKARYOTES
In eukaryotic cells, three distinct RNA polymerases—
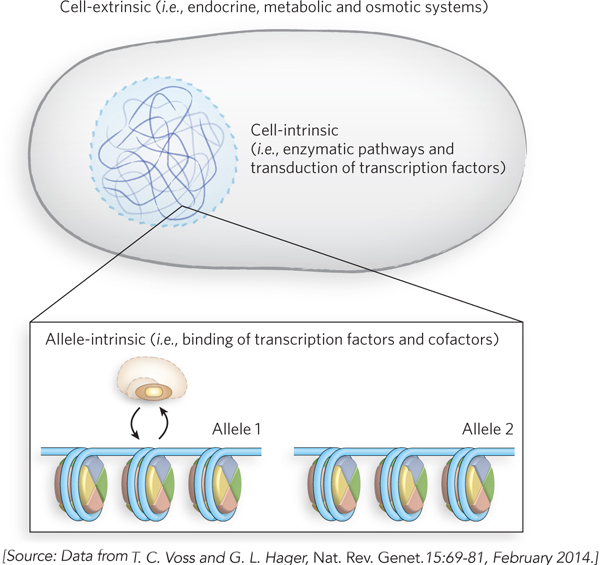
HIGHLIGHT 15-2 MEDICINE: Using Transcription Factors to Reprogram Cells
Patterns of gene transcription largely control how cells develop into specific cell types. This process is of great importance in medicine, because the possibility of reprogramming cells to carry out specific functions could revolutionize the treatment of patients with degenerative diseases. Experimental attempts to reprogram cells began several decades ago with the discovery that engineering of an oocyte (egg cell) to contain the nucleus of an adult cell can cause the nucleus to revert to an undifferentiated state. This process, called somatic cell nuclear transfer (SCNT), can produce an embryo and embryonic stem cells with the genetic makeup of an adult cell. Presumably, these results come about through the reprogramming of transcription in the composite cells.
This idea was tested and validated in 2006, when researchers found that fibroblasts can be induced to undergo a dramatic cell-
To test this possibility, Marius Wernig and his colleagues at Stanford University set out to convert mouse fibroblasts into neurons. Reasoning that multiple transcription factors were probably necessary to reprogram fibroblasts to a neuronal fate, the researchers cloned 19 genes that encode transcription factors expressed specifically in neural tissues or function during neural development. The genes were cloned into lentiviruses, viral vectors that could be used to introduce the genes into mouse fibroblasts by infection. To detect changes in cell fate, the researchers used fibroblasts derived from mouse embryos and tail tips of newborn or adult mice that had been genetically altered to express a green fluorescent protein marker when the gene for the protein Tau was turned on. Because the Tau gene is specifically expressed in neurons, cells that had acquired at least this property of neurons—
When all 19 of the candidate transcription factors were introduced into the fibroblasts, some of the cells turned green. By a process of elimination, the researchers eventually found that a combination of only three transcription factors was sufficient to convert fibroblasts into neurons (Figure 1). These factors—
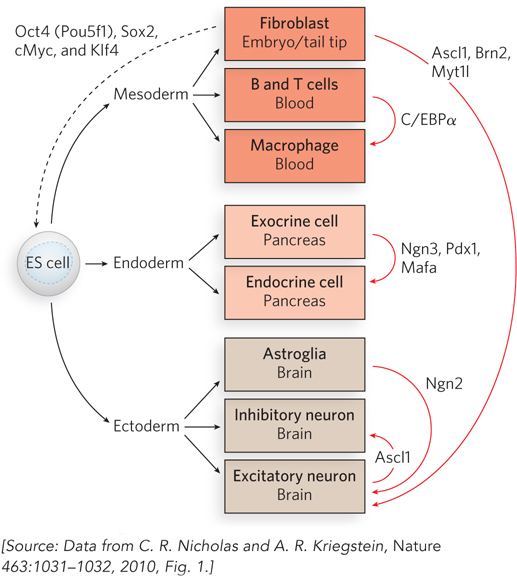
Beyond its implications for understanding transcriptional activation and regulation, this discovery offers the intriguing possibility of creating cell types at will. If such transcription-
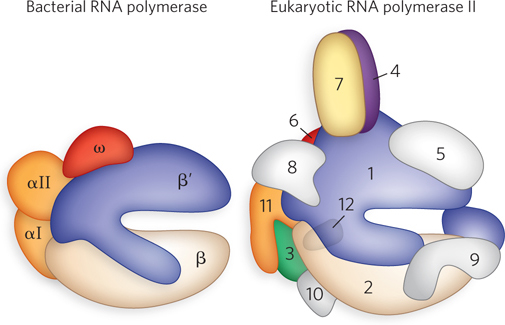
Because transcription is a fundamental process in all cells, it is not surprising that some eukaryotic RNA polymerase subunits are homologous to those of bacterial polymerase. Furthermore, some subunits are common to all three of the eukaryotic polymerases (see Table 15-1). Relative to bacteria, eukaryotes require additional factors to help RNA polymerases find and access promoters in the cell nucleus. This is because eukaryotic DNA is packaged into chromatin through the formation of nucleosomes (see Chapter 10). In addition, the sheer size of eukaryotic genomes, and the large number of promoters to be sorted through, probably requires additional transcription machinery.
We begin with a brief discussion of all three eukaryotic polymerases and their promoters, and then focus on Pol II transcription. As the polymerase responsible for transcribing the genes that encode proteins, Pol II is the most extensively studied of the three eukaryotic polymerases.
Eukaryotic Polymerases Recognize Characteristic Promoters
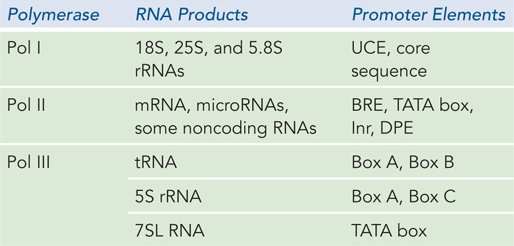
Each of the three types of RNA polymerase that make up the eukaryotic transcription machinery transcribes only certain classes of genes, and thus each type binds to specific and distinct promoter sequences. Pol I binds to a single type of promoter that controls the expression of the pre-
Although each polymerase works with its own unique set of transcription factors, all three types use a factor called the TATA-
538
RNA Polymerase I Promoters Synthesis of pre-

539
The number of rRNA genes varies among organisms. Pol I promoter sequences also vary, but within a species, all Pol I promoters are the same. Low levels of transcription can be observed in the presence of a preinitiation complex comprising Pol I and selectivity factor 1 (SL1), which is a complex of TBP and three TAFs. Higher levels of transcription require, in addition to Pol I and SL1, an upstream binding factor (UBF). UBF binds to both the UCE and the core promoter and to SL1, stabilizing the complex with Pol I and helping recruit the polymerase to the promoter.
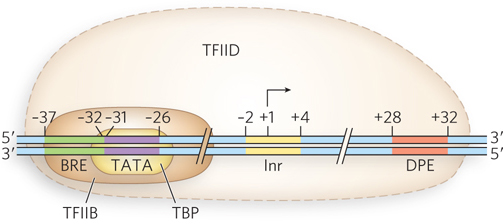
RNA Polymerase II Promoters Many Pol II promoters share certain sequence features, including a TATA box near −30 and an initiator sequence (Inr) near the RNA start site at +1 (Figure 15-20). Pol II promoters also sometimes include a sequence upstream from the TATA box, called a TFIIB recognition element (BRE), and a sequence downstream from the initiator, the downstream promoter element (DPE). These sequences comprise the core promoter. Other sequences are also needed for efficient Pol II recognition and transcription in the cell, such as upstream promoter elements and enhancers. These regulatory sequences, which can be located many thousands of base pairs away from the promoter they influence, bind a variety of specific transcription factors that either activate or repress transcription, depending on various stimuli. The proteins that bind to the elements in the promoter are discussed shortly.
KEY CONVENTION
The nomenclature for transcription factors indicates which RNA polymerase is involved. TFII is a transcription factor for RNA polymerase II, and TFIII is a transcription factor for RNA polymerase III. Individual factors are distinguished by an appended A, B, C, and so on (e.g., TFIIA, TFIIIB).
540
RNA Polymerase III Promoters Pol III is the largest RNA polymerase with the greatest number of subunits. All of its transcription products are short, untranslated RNAs, most less than 300 nucleotides long. In addition to 5S rRNA, they include tRNAs; 7SL RNA, which is required for introducing proteins into membranes as part of the signal recognition particle (see Chapter 18); and several RNAs involved in mRNA, tRNA, and rRNA processing. Perhaps reflecting their varied gene products, Pol III promoters differ in sequence and in components. The promoters of tRNA genes include two segments, Box A and Box B, located a short distance apart within the tRNA-
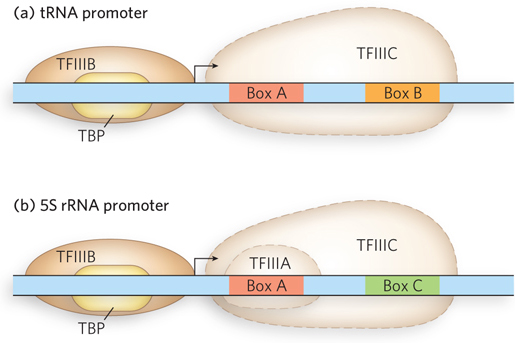
Like the other eukaryotic polymerases, Pol III requires transcription factors. The tRNA genes require TFIIIB and TFIIIC, whereas the 5S rRNA gene requires TFIIIB, TFIIIC, and TFIIIA. Transcription of tRNA genes begins when TFIIIC binds to the promoter boxes within the gene, and then recruits TFIIIB. TFIIIB includes TBP and recognizes the DNA just upstream from the transcription start site. Together, these factors recruit Pol III to the transcription start site; TFIIIC is transiently displaced as the polymerase transcribes through its binding site in the DNA. In 5S rRNA transcription, TFIIIA binds to the DNA within the transcribed region and helps recruit TFIIIC.
Pol II Transcription Parallels Bacterial RNA Transcription
Pol II–
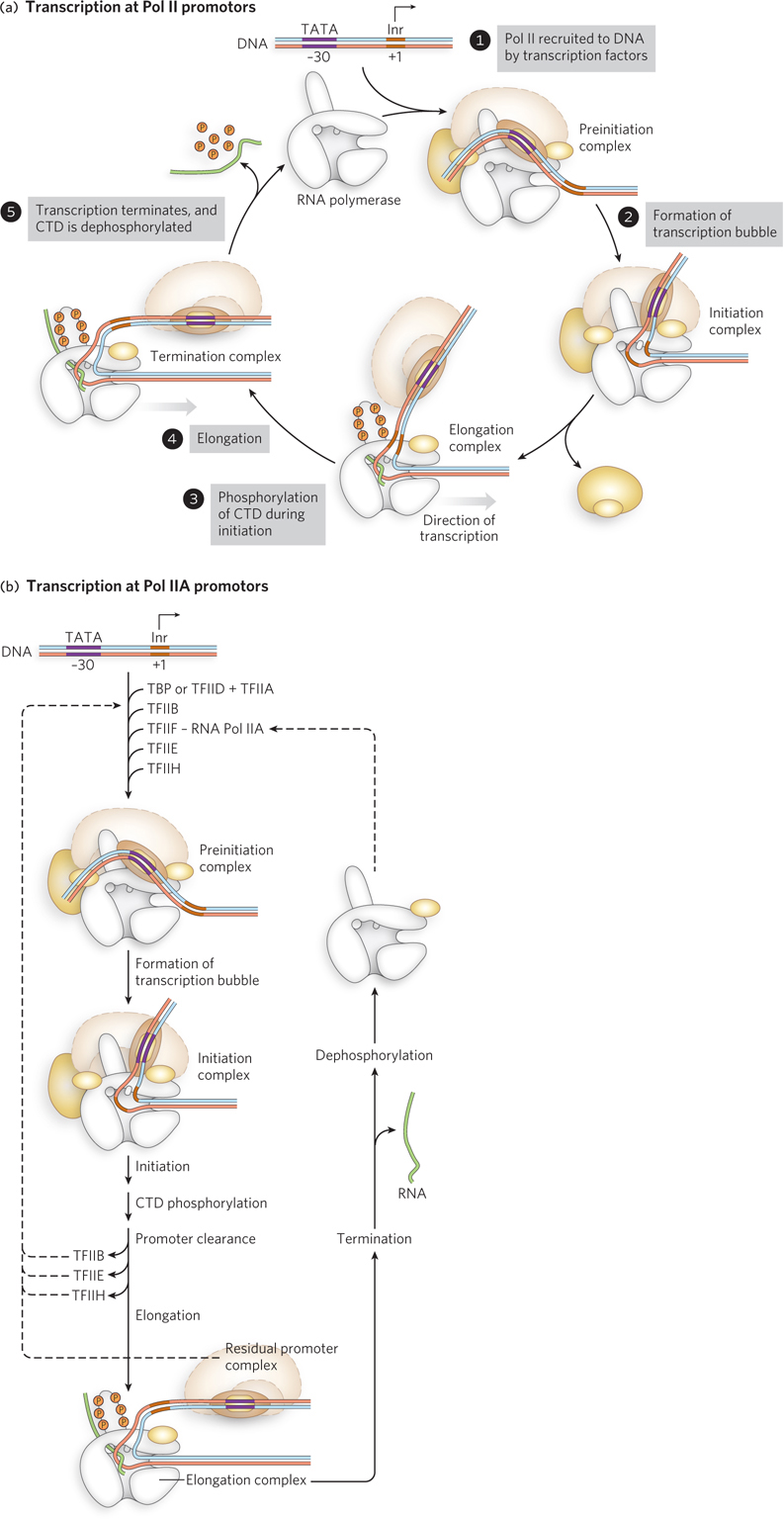
An overview of the Pol II transcription complex is shown in Figure 15-23. Playing a role much like that of sigma factors in helping bacterial RNA polymerase recognize and bind promoter sequences, general transcription factors associate with promoter DNA and recruit Pol II to form a preinitiation complex (Figure 15-23a, step 1). The preinitiation complex is converted to an initiation complex by unwinding the DNA (step 2). During initiation (step 3), the C-
Transcription Factors Play Specific Roles in the Transcription Process


The transcription initiation mechanism has been most extensively studied for Pol II. Recruitment begins with binding of the TATA box by TFIID. Like many transcription factors, TFIID is a multiprotein complex, which includes TBP and TAFs. The TAFs fine-
541
542
The discovery of TBP and its importance as a general transcription factor required for all transcription by Pol II raised questions about how and why it binds so specifically to the TATA element. This mystery of the transcription initiation process was solved when the research groups of Paul Sigler and Stephen Burley independently determined the molecular structure of TBP bound to DNA, using x-
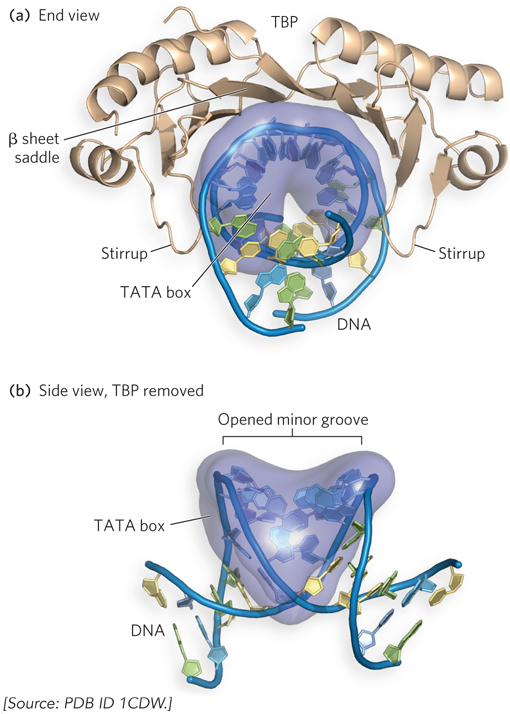

In addition to TBP, Pol II requires an array of transcription factors to form an active transcription complex. The general transcription factors required at every Pol II promoter are highly conserved in all eukaryotes. Using cell-
543
Once assembled on a promoter, Pol II typically produces a few abortive transcripts before entering the elongation phase of transcription—
The CTD, part of the largest polymerase subunit, consists of multiple repeats of the seven amino acid sequence Tyr-
TFIIE and TFIIH are released during synthesis of the initial 60 to 70 nucleotides of RNA, as Pol II enters the elongation phase of transcription. Notably, phosphorylation of Pol II CTD also influences downstream processing of the RNA transcript, providing a mechanism for coupling transcription to RNA splicing and intracellular transport (as discussed in Chapter 16).
In the elongation phase, polymerase activity is greatly enhanced by elongation factors. They suppress pausing during transcription, enhance polymerase editing of misincorporated bases by hydrolysis (as for bacterial RNA polymerase), and recruit protein complexes involved in posttranscriptional processing of the mRNA. Once the RNA transcript is completed, transcription is terminated. In eukaryotes, termination is often triggered by endonucleases that recognize and cleave specific sequences in the newly synthesized RNA, leading to disassembly and dissociation of the transcription complex. Pol II is then dephosphorylated and recycled, readying it to initiate another transcript (see Figure 15-23).
Transcription Initiation In Vivo Requires the Mediator Complex
As we have seen, the initiation and control of eukaryotic mRNA synthesis requires a large set of evolutionarily conserved general transcription factors that function at most, if not all, genes. These include initiation factors TFIIB, TFIID (which includes the TATA-
Mediator functions as an intermediary between specific transcription factors bound at upstream promoter elements or enhancers and the Pol II complex and general initiation factors bound at the core promoter (Figure 15-25a). First discovered and purified from yeast by Roger Kornberg and his colleagues, Mediator was found to be required for transcriptional activation by specific activators in vitro, using a reconstituted enzyme system containing purified Pol II and general initiation factors. Yeast Mediator has 20 subunits in three distinct subdomains, referred to as the head, middle, and tail modules (Figure 15-25b). An additional module, which includes a kinase enzyme complex, is associated with a subset of yeast Mediator complexes. The presence of the kinase corresponds to repression of a subset of genes, suggesting a role for Mediator in transcriptional down-
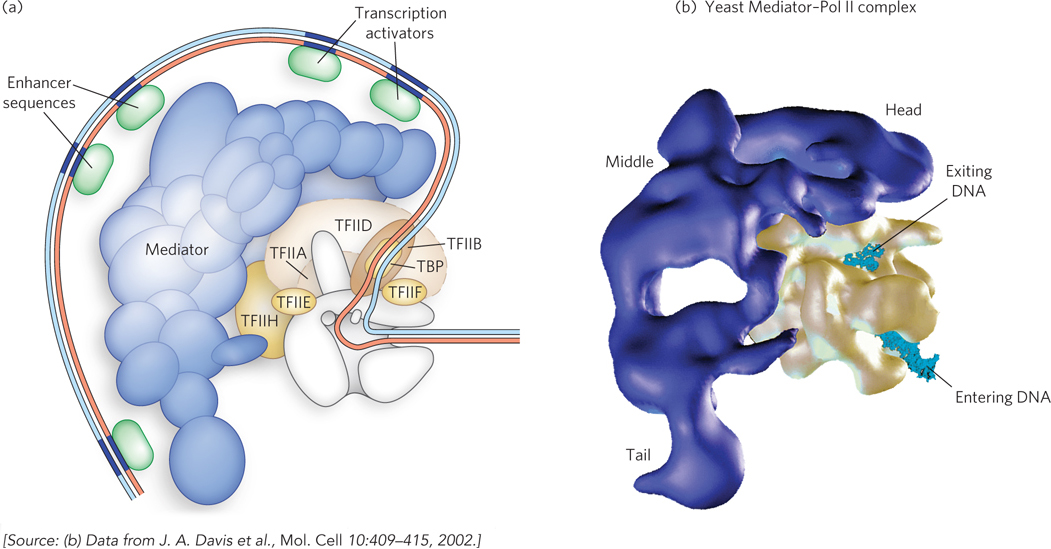
Human Mediator contains a set of consensus subunits similar to those in yeast. As in yeast, multiple forms of Mediator seem to function differently in the transcriptional control of different sets of genes. In particular, the kinase module can exert a repressive effect when associated with the mammalian Mediator, whereas other auxiliary proteins are associated with an activating form of Mediator.
The mechanisms by which Mediator complexes control mRNA synthesis involve direct interactions with DNA-
544
Termination Mechanisms Vary among RNA Polymerases
The three eukaryotic RNA polymerases use different strategies for terminating transcription, although these mechanisms have some aspects in common. The Pol III and Pol I termination pathways seem to be simpler than the Pol II pathway. Pol III terminates transcription at T-
In contrast, Pol II termination does not occur at a conserved site or at a constant distance from the 3′ end of mature RNAs. In mammals, it takes place anywhere from a few base pairs to several kilobase pairs downstream from the 3′ end of the mature transcript. The 3′ end of the mature mRNA includes a stretch of A nucleotides, called a poly(A) tail, that is essential for translation into protein (see Chapter 18). A polyadenylation signal sequence (typically AAUAAA) is present in the primary transcript (and directly encoded by the DNA). Factors responsible for cleavage of the primary transcript bind to the AAUAAA sequence, resulting in cleavage somewhat downstream from that position. Only after this cleavage is the poly(A) tail added. Pol II termination is coupled to 3′-end processing of precursor mRNA transcripts, and the intact polyadenylation signal is necessary for termination of transcription of protein-
Two different models have been proposed to explain how 3′-end processing contributes to Pol II transcription termination. The first, known as the allosteric or antiterminator model, proposes that transcription through the poly(A) site triggers conformational changes in the Pol II elongation complex caused by the dissociation of elongation factors and/or association of termination factors. This is analogous to the hairpin model of termination in bacteria (see Section 15.2). According to the allosteric model, these conformational changes in Pol II cause it to fall off the DNA template. The second model, the torpedo model, suggests that after mRNA synthesis is complete, Pol II remains associated with the DNA template and continues the transcription reaction to extend the 3′ end of the mRNA. Protein complexes cleave the mRNA at the polyadenylation site, producing a new 3′ end that can be recognized and extended by the enzyme poly(A) polymerase. The new 5′ end of the downstream, or residual, mRNA strand becomes a substrate for an enzyme called Xrn2, a 5′→3′ exonuclease (an exoribonuclease) that attaches to the CTD of Pol II (Figure 15-26). Xrn2 proceeds to degrade the uncapped residual RNA in the 5′→3′ direction until it reaches Pol II. Similar to the ρ factor in ρ-dependent termination in bacteria, Xrn2 triggers dissociation of Pol II by either pushing the polymerase off the DNA template or pulling the template out of the RNA polymerase.
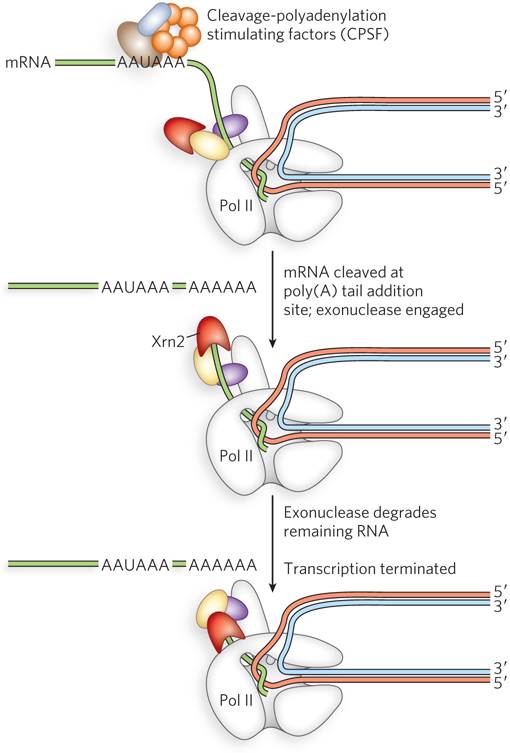
545
Transcription Is Coupled to DNA Repair, RNA Processing, and mRNA Transport
In eukaryotes, transcription is coupled to other activities, including the repair of damaged DNA and various kinds of RNA processing and transport events. Researchers noticed that DNA damage repair and mRNA processing and transport are more efficient for genes that are actively being transcribed. Furthermore, DNA lesions in the template strand are repaired somewhat more efficiently than lesions in the coding (nontemplate) strand. For DNA repair, these remarkable observations are explained by the alternative functions of the TFIIH subunits. Not only does TFIIH participate in forming the closed complex during assembly of a transcription complex, but some of its subunits are also essential components of the separate nucleotide excision repair complex (see Chapter 12). When Pol II transcription stalls at the site of a DNA lesion, TFIIH reassociates with the DNA and transcription machinery. TFIIH can then interact with the lesion and recruit the entire nucleotide excision repair complex. Mutations causing deletion of certain TFIIH subunits produce human diseases. Two examples are xeroderma pigmentosum, with its associated photosensitivity and tumor susceptibility, and Cockayne syndrome, which is characterized by arrested growth, photosensitivity, and neurological disorders.
Eukaryotic mRNA is processed in a variety of ways before it is shipped across the nuclear membrane to the cytoplasm for translation. We discuss the mechanisms of these processing events in Chapter 16, but it is important to note here that like DNA repair, mRNA processing is naturally linked to transcription. This is possible because some of the same proteins required for elongating RNA transcripts are also required for 5′-end processing (5′ capping) of the RNA. Because these activities are coupled, transcripts can be processed as they are synthesized.
SECTION 15.3 SUMMARY
The RNA polymerases of eukaryotes (Pol I, II, and III) share some structural and functional features with bacterial RNA polymerase, but they are much larger and require additional proteins—
transcription factors— to begin efficient transcription at promoter sequences. Pol I, II, and III recognize distinct promoter sequences and require unique sets of transcription factors, with the exception of TATA-
binding protein (TBP), which is used by all three polymerases. As in bacteria, transcription initiation in eukaryotes is highly regulated and includes multiple steps that lead to assembly of an active polymerase complex at a promoter. Pol II transcription (the most studied) proceeds through distinct phases of assembly, initiation, elongation, and termination.
In eukaryotes, the Pol II C-
terminal domain must be phosphorylated before transcription can proceed from initiation to elongation. Transcriptional regulation in eukaryotes is enhanced by Mediator, a large protein complex that binds simultaneously with general transcription factors associated with Pol II and specific transcription factors associated with upstream promoter elements.
546
Two hypotheses for transcription termination suggest a role for mRNA sequence elements and for an exonuclease, respectively.
TFIIH, a eukaryotic transcription initiation factor, can start nucleotide excision repair of DNA when Pol II encounters a lesion in the template strand. Transcription and processing of mRNA are coupled, because some Pol II transcription factors are also required for pre-
mRNA processing events.
UNANSWERED QUESTIONS
Many details of transcription mechanisms are known, but future challenges include discovering how, where, and when transcripts are made and how they are used in cells for functions beyond encoding and synthesizing proteins.
How does RNA polymerase coordinate with other enzymes and regulators during gene expression? The pausing of RNA polymerase during transcription is thought to help the enzyme coordinate with other steps in the protein-
producing pathway. How does this work, and do proteins such as RNA- modifying enzymes recognize paused transcripts as substrates? Understanding these mechanisms and how they differ in bacteria and humans will provide basic information about transcription and help define steps that could be disrupted to block bacterial growth, thus serving as good antibacterial drug targets. What is the mechanism of promoter sequence recognition? Are there other promoter sequences that haven’t yet been identified? These questions are especially relevant given the explosion in numbers of non-
protein- coding RNA transcripts produced by Pol II that are now being discovered. Pol II seems to transcribe much of the human genome at low levels. How is the polymerase recruited to the DNA for this purpose? Perhaps Pol II uses its weak, nonspecific DNA- binding affinity, or perhaps it can transcribe past termination signals at some frequency. How is such transcription controlled? How exactly is transcription terminated? This is still not well understood, especially in eukaryotes. If this process were better understood, it might be possible to exploit it for therapeutic purposes, such as inducing early termination of viral transcripts.
547
RNA Polymerase Is Recruited to Promoter Sequences
Dynan, W.S., and R. Tjian. 1983. Isolation of transcription factors that discriminate between different promoters recognized by RNA polymerase II. Cell 32:669–
Dynan, W.S., and R. Tjian. 1983. The promoter-

One of the first experiments to demonstrate how promoters are recognized by RNA polymerase II involved separating the contents of cultured human HeLa cells into the components required for accurate gene transcription in vitro. Bill Dynan and Bob Tjian used this system to find that Sp1 is a promoter-

Further experiments using deletion mutants of the SV40 promoter showed that transcriptional activation by Sp1 required sequences within tandem 21 bp repeats located 70 to 110 bp upstream from the transcription initiation site. DNA footprinting revealed that DNA sequences within the 21 bp repeat region were bound by Sp1 (Figure 2). In this experiment, SV40 promoter-
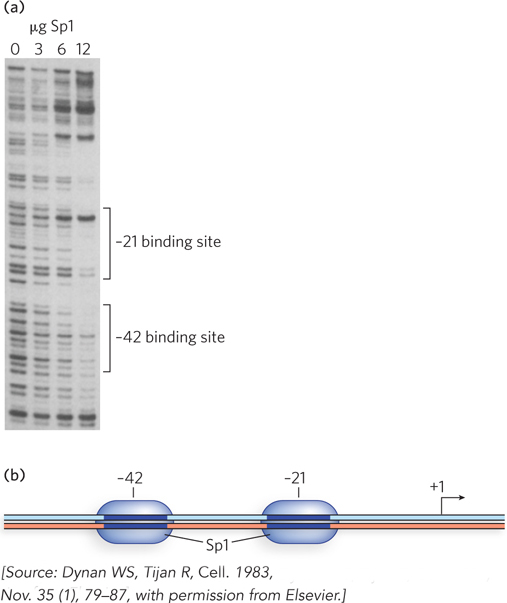
This was an exciting result, because it indicated the presence of a specific site for Sp1 binding. Furthermore, there was a correlation between this promoter-
548
RNA Polymerases Are Both Fast and Slow
Neuman, K., E. Abbondanzieri, R. Landick, J. Gelles, and S.M. Block. 2003. Ubiquitous transcriptional pausing is independent of RNA polymerase backtracking. Cell 115:437–
Shaevitz, J.W., E.A. Abbondanzieri, R. Landick, and S.M. Block. 2003. Backtracking by single RNA polymerase molecules observed at near-
Molecular biologists have noticed that rather than transcribing DNA at a constant pace, an RNA polymerase hesitates at certain sites as it moves along the template. However, because the individual polymerases in a solution are not synchronized, the kinetics of pausing are difficult to study.
To circumvent this problem, Stephen Block, Bob Landick, and their coworkers chemically attached transcription elongation complexes to polystyrene beads, one polymerase to a bead. They used antibodies to attach one end of the template DNA to the surface of a microscope stage (Figure 3a), and used a laser trap to keep the bead (and RNA polymerase) in a fixed position while moving the stage (and the DNA) away, pulling the DNA taut through this constant force. They monitored the motion of the bead (and polymerase) with respect to the stage surface as the DNA was threaded through the elongation complex. This system was used to assess the force on the bead required to counteract the motion of the RNA polymerase. From these measurements, pause and arrest sites on the DNA could be mapped, and the maximal speed reached by RNA polymerase between two pause sites was measured.
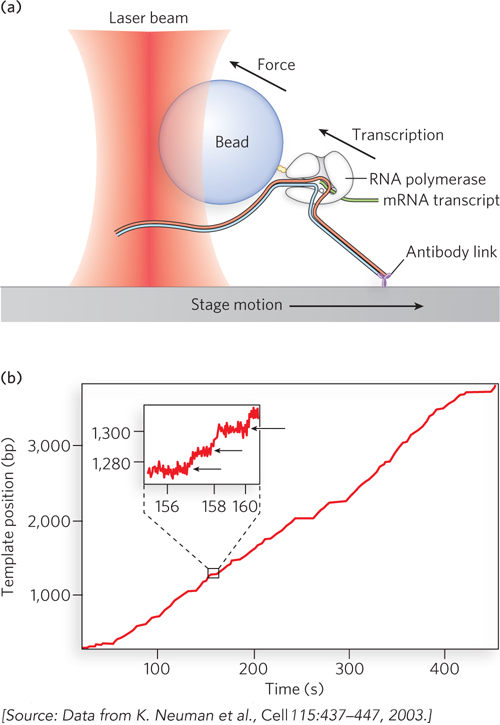
Although the experiments were conducted on single molecules, thousands of recordings were made, enabling the investigators to compare individual polymerase complexes. The results showed that RNA polymerase molecules alternate between constant-
549