16.5 RNA CATALYSIS AND THE RNA WORLD HYPOTHESIS
Study of the posttranscriptional processing of RNA molecules led to one of the most exciting discoveries in modern molecular biology: the existence of ribozymes, enzymes consisting of RNA. Some introns found in rRNA, mRNA, and tRNA genes in bacteria, mitochondria, chloroplasts and bacteriophages have intrinsic self-splicing activity, meaning that no proteins are required to catalyze their excision from precursor transcripts. In addition, in some types of cells, certain other RNA processing reactions, such as the removal of short sequences from the precursors of tRNAs, are catalyzed by RNA. RNA is the only known molecule that is capable of both encoding genetic information and functioning as an enzyme. For this reason, many scientists have proposed that RNA could have formed the basis for the early life forms that evolved into modern organisms, because, in principle, RNA could both carry and copy genetic information. We discuss here the properties of RNA that support its multiple functions and explore the implications of the RNA world hypothesis.
Ribozymes Catalyze Similar Kinds of Reactions But Have Diverse Functions
The catalytic RNAs discovered thus far include self-splicing introns, bacterial RNase P, and several additional classes of naturally occurring ribozymes. Experimentally, it has proved possible to select catalytically active RNA molecules from pools of randomized sequences prepared in the laboratory. Ribozymes selected in this way can have a variety of enzymatic functions, demonstrating the inherent catalytic capabilities of RNA. The activities of many ribozymes, natural and selected in the laboratory, consist of breaking or joining phosphodiester bonds in RNA substrates. In some cases, such as self-splicing introns, the breaking and joining are coupled, whereas other ribozymes, such as RNase P, catalyze bond cleavage only.
Because many ribozymes act on an RNA substrate, often a part of the ribozyme itself, base-pairing interactions are critical for binding and positioning the substrate for reaction. Crystallographic structures of ribozymes and their components, determined over the past two decades, show that base pairing is also central to the architecture of ribozyme active sites. The first crystal structure of a large folded RNA, the P4-P6 domain of the Tetrahymena group I self-splicing intron (see Figure 16-14), revealed how base-paired RNA helices can pack together through non-Watson-Crick interactions and specifically positioned Mg2+ ions.
The structural patterns observed in the P4-P6 RNA, including the extensive use of unpaired A residues in the stabilizing, noncovalent contacts essential to the three-dimensional structure, have been observed repeatedly in RNAs ranging from small, self-cleaving ribozymes to the RNAs of ribosomes. Thus, RNA molecules use weak interactions, including the hydrophobic interactions and hydrogen bonding inherent in base pairing and base stacking, along with site-specific metal ion coordination, to form a wide variety of structures with ligand-binding sites and catalytic centers. DNA molecules are inherently less able to form such stable three-dimensional structures, due to subtle but crucial differences in the geometry of the phosphodiester backbone and the lack of a 2′ hydroxyl in DNA nucleotides. RNA is therefore uniquely positioned in biology not only to encode genetic information but also to modify it, and possibly even to replicate it.
The known repertoire of ribozymes continues to expand. Some virusoids, small RNAs associated with plant RNA viruses, include a structure that promotes a self-cleavage reaction. (Highlight 16-3 describes the self-cleaving ribozyme of a human virus.) The hammerhead ribozyme is in this class, catalyzing the hydrolysis of an internal phosphodiester bond important for producing unit-length virusoid RNAs. In the eukaryotic spliceosome, the splicing reaction requires a catalytic center formed, at least in part, by the U2, U5, and U6 snRNAs. And perhaps most importantly, an RNA component of ribosomes catalyzes the synthesis of proteins (see Chapter 18).
HIGHLIGHT 16-3 EVOLUTION: A Viral Ribozyme Derived from the Human Genome?
Ribozymes are thought to have been important in early evolution, because they provide the potential to both store and replicate genetic information within the same molecule. However, the age and origin of the known, naturally occurring ribozymes are not easy to determine.
Experiments by Jack Szostak and his colleagues at Harvard Medical School showed that, at least in one case, ribozyme evolution occurred relatively recently. The research group was looking for self-cleaving ribozymes in the human genome. Total human cellular RNA was isolated and tested for the ability to generate shorter fragments in a Mg2+-dependent reaction. Cleaved fragments were selectively enriched and retested, and after many such cycles, the researchers isolated RNAs that could catalyze autocleavage at specific sites. In an experiment with one of these RNAs, the starting RNA fragment was found to cleave itself over the course of several hours, resulting in shorter fragments that could be separated by denaturing polyacrylamide gel electrophoresis (Figure 1).
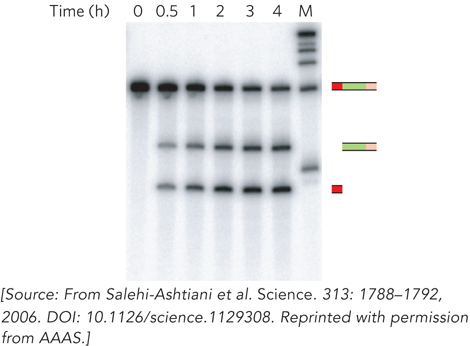
FIGURE 1 Gel revealing a viral-like ribozyme in human genomic RNA. Samples of the reaction mixture containing the transcript with the ribozyme sequence and flanking sequences were removed for analysis at different time intervals. Over time, the transcript was processed into shorter fragments corresponding to ribozyme-catalyzed strand cleavage at the junction between the 5′ flanking sequence and the boundary of the ribozyme sequence (shown in the representations to the right of the gel). M indicates a marker lane.
Remarkably, the ribozyme discovered in this experiment is structurally and biochemically related to ribozymes first discovered in the human hepatitis delta virus (HDV). Furthermore, sequence comparisons showed that the ribozyme occurs only in mammals, implying that it may have evolved as recently as 200 million years ago. HDV itself may have arisen sometime later, forming from fragments of human RNA.
Ribozymes vary greatly in size. Ribosomal RNA is thousands of nucleotides long, the Tetrahymena self-splicing group I intron contains ∼400 nucleotides, and the smallest active hammerhead ribozyme of virusoids consists of two RNA strands with only 41 nucleotides in all. Experiments have shown that in each case, a ribozyme can be inactivated by heating above its melting temperature or by the addition of denaturing chemicals or complementary oligonucleotides, which disrupt normal base-pairing patterns. Furthermore, ribozymes can be inactivated if essential nucleotides are changed, forming the basis for many experiments demonstrating the importance of particular nucleotides or base-pairing interactions in ribozyme function (see the How We Know section at the end of this chapter).
Could RNA Have Formed the Basis for Early Life on Earth?
The existence of so many kinds of ribozymes has fueled debate about why these catalysts occur in nature and what they might indicate about the origin of enzymes. Without catalysts, life would not be possible, and an understanding of how enzymes evolved is central to our understanding of life’s origins.
The RNA world hypothesis proposes that organisms comprised entirely or mostly of RNA evolved on early Earth. The base-pairing properties of RNA could have been used to store information, as occurs today in many viruses, and the capacity of RNA to form a variety of structures lent itself to the emergence of ribozymes and perhaps other functional molecules, such as those forming membrane-spanning pores. Using in vitro selection methods, Gerald Joyce and his colleagues found that even RNAs containing just two kinds of nucleotides that can base-pair with each other can become catalytically active. This observation indicates that simple nucleic acids that might have been around before life began could have given rise to activities necessary for template-dependent replication.
Eventually, DNA supplanted RNA as a data storage molecule because of its greater chemical stability. Proteins, with broader catalytic capabilities stemming from chemically diverse amino acids, became the specialized catalytic molecules. The few remaining ribozymes are remnants of the RNA world and provide clues to its former existence.
Several aspects of RNA chemistry and behavior support the RNA world hypothesis. These include the findings that RNA can function as both a genome and an enzyme, that RNA catalyzes peptide bond formation on ribosomes and thus is responsible for protein synthesis, and that components of RNA form spontaneously in “prebiotic soup” experiments designed to replicate conditions on early Earth. Furthermore, the continuing discovery of RNA molecules that function in fundamental aspects of gene expression and regulation underscores the pervasive and presumably ancient roles of RNA in virtually all aspects of life. More difficult to explain is how and why the specific sugars found in RNA and DNA were selected under prebiotic conditions, and how nucleotides could have been assembled and polymerized without the assistance of enzymes. Researchers continue to investigate these issues, using RNA and related polymers. At present, the RNA world hypothesis is considered to be the most likely explanation for the emergence and evolution of modern organisms.
SECTION 16.5 SUMMARY
Ribozymes are important for catalyzing several RNA processing reactions, including self-cleavage of viral RNA replication intermediates and precursor tRNA processing.
The RNA world hypothesis, based on the special properties of RNA that enable it to form stable functional structures and encode genetic information, postulates that RNA-based life predated modern DNA-based organisms.
UNANSWERED QUESTIONS
The study of RNA processing reactions has been a long-standing and active area of research, yet much remains to be deciphered.
How did introns evolve and how have they achieved their current roles in biology? We don’t yet know why there are introns and whether they are ancient or more recent acquisitions in genes. Some introns have been found to encode regulatory RNA molecules that function in the processing of rRNA and in the control of gene expression levels (see Chapter 22). Whether these regulatory RNAs are a cause or a result of the presence of introns is not known. Although the origin of introns may remain uncertain, further insights about their roles in the continuing evolution of genomes will be illuminating and may shed light on diseases that result from inaccurate intron removal and processing.
How does alternative splicing work? Experimental methods, including microarray technology and genome-wide transcript sequencing, have revealed an abundance of alternative splicing in mammalian cells. However, the mechanics of such molecular gymnastics have yet to be fully determined. Future research will focus on how particular splice sites are chosen, the frequency with which genes are alternatively spliced, and the roles of splicing regulation in disease.
How do ribozymes contribute to modern biology? We don’t have clear information on the origin and maintenance of ribozymes in particular biological niches. Some researchers have proposed that the spliceosome is a ribozyme, but this remains unproven. Current evidence suggests that the catalytic center of the spliceosome may in fact include both RNA and protein components. If true, such close association of RNA and protein would provide the first example of a true ribonucleoprotein enzyme linking RNA-based and protein-based catalysts.
What governs the assembly and disassembly of P bodies? Do the proteins that localize to P bodies function there, or are they held on standby until needed elsewhere in the cell?
Studying Autoimmunity Led to the Discovery of snRNPs
Lerner, M.R., and J.A. Steitz. 1979. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc. Natl. Acad. Sci. USA 76:5495–5499.

Joan Steitz
The story of the discovery of snRNPs highlights the serendipity inherent in scientific research. In the early 1980s, Joan Steitz and her colleagues at Yale University were investigating the molecular basis of the puzzling autoimmune disease systemic lupus erythematosus, which causes a red facial rash, fatigue, and arthritis, among other symptoms. Working with partially purified antibodies isolated from the blood of lupus patients, Steitz discovered that the antigens—the binding targets—of these antibodies are normal cellular particles containing a single small nuclear RNA complexed with proteins: snRNPs. Specifically, the autoantibodies associated with lupus recognize a set of snRNP proteins that were eventually named Sm, for the last name of the woman (Smith) whose serum samples were tested.
The snRNPs isolated by precipitation with anti-Sm antibodies were found to bind preferentially to RNAs containing intron-exon junctions, suggesting the involvement of these snRNPs in pre-mRNA splicing. In this experiment, nuclei isolated from cultured human cells were treated with radiolabeled UTP so that any newly synthesized RNA would incorporate the radiolabel. The nuclei were then incubated with anti-Sm antibodies under conditions in which the antibodies could enter the nucleus. After incubation for an hour, RNA was purified from the nuclei and fractionated by size, using agarose gel electrophoresis. The radiolabeled pre-mRNAs and mature RNAs (i.e., unspliced and spliced RNAs, respectively) were identified with specific DNA probes, and the relative amount of each was quantified based on the amount of radioactivity in each band in the gel. Radioactivity was plotted as a function of fraction number, which correlates with mRNA size (Figure 1). In the absence of added anti-Sm antibodies, both unspliced and spliced mRNAs were detected (top graph). In contrast, the presence of anti-Sm antibodies inhibited the production of spliced mRNAs, such that only a peak corresponding to unspliced pre-mRNAs was detected (bottom graph).
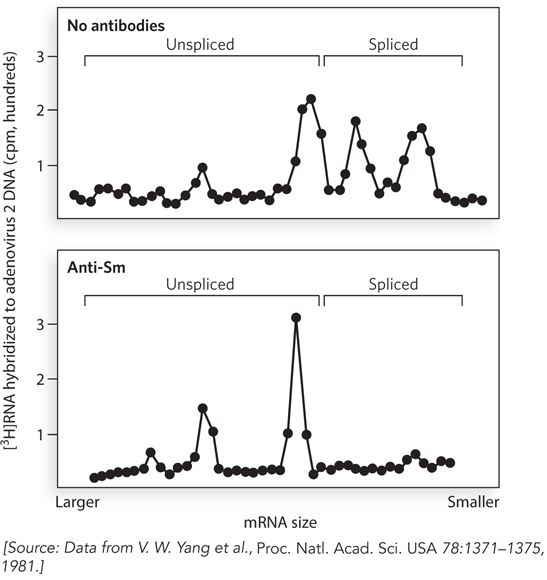
FIGURE 1 Results of labeling experiments showing that processing of pre-mRNA is blocked by anti-Sm antibodies. In the absence of antibodies (upper panel), multiple fractions corresponding to spliced mRNAs are detected; in the presence of antibodies (lower panel), only larger, unspliced pre-mRNAs are detected.
These data led to further studies that elucidated the specific roles of snRNPs in recognizing and catalyzing the removal of introns in the pre-mRNAs of all eukaryotic cells. This latter discovery was recognized by the Nobel Prize in Medicine, awarded to Phillip Sharp and Richard Roberts in 1993. We still don’t know how Sm proteins can induce an autoimmune response, given that, presumably, they are sequestered within snRNPs in the nucleus.
RNA Molecules Are Fine-Tuned for Stability or Function
Cerutti, P., J.W. Holt, and N. Miller. 1968. Detection and determination of 5,6-dihydrouridine and 4-thiouridine in transfer ribonucleic acid from different sources. J. Mol. Biol. 34:505–518.
Hughes, D.G., and B.E. Maden. 1978. The pseudouridine contents of the ribosomal ribonucleic acids of three vertebrate species: Numerical correspondence between pseudouridine residues and 2′-O-methyl groups is not always conserved. Biochem. J. 171:781–786.
Kuchino, Y., and E. Borek. 1978. Tumour-specific phenylalanine tRNA contains two supernumerary methylated bases. Nature 271:126–129.
Unusual and chemically modified nucleotides in tRNAs, rRNAs, and a few other kinds of RNA molecules were discovered when these RNAs were purified from cells in sufficient quantity for classical analysis by thin-layer chromatography—a standard technique for biochemists. In this method, RNA is hydrolyzed to its single-nucleotide components by ribonucleases, and the digestion products are applied to a glass or plastic plate coated with a thin layer of a chemical that absorbs liquid. One edge of the plate is placed in a solvent that is slowly absorbed upward through the surface layer (Figure 2). As the solvent moves through the sample and up the plate, different nucleotides in the sample move at different rates based on their solubility and their affinity for the surface material. The technique allows clean separation of A, G, C, and U nucleotides. Any chemically modified or noncanonical nucleotides that appear as extra “spots” on the plate can be scraped off for analysis by methods such as mass spectrometry.
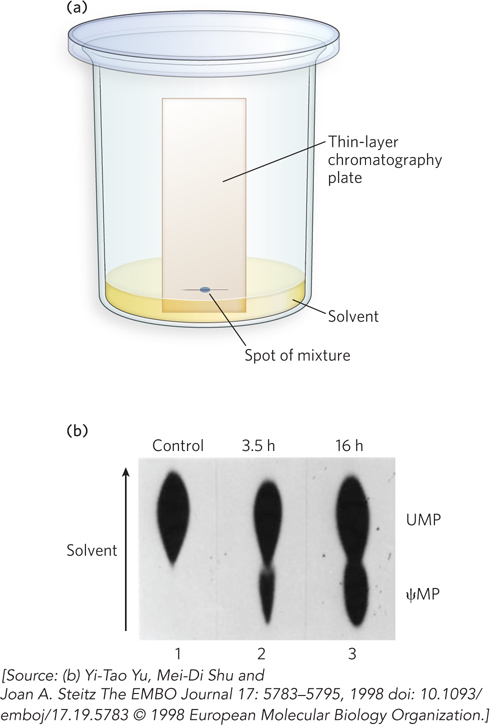
FIGURE 2 Thin-layer chromatography to detect chemically modified nucleotides. (a) RNase-digested tRNA is spotted onto a silica-coated glass plate, and solvent and nucleotides migrate up the plate by capillary action. (b) Modified and unmodified nucleotides—in this case, pseudouridine monophosphate (ψMP) and UMP, as control—migrate at different rates, based on their different solubilities in the solvent and their different affinities for the silica gel.
In this way, investigators initially discovered that tRNAs contain a few nucleotides other than the standard four. How are such unusual bases synthesized, and why are they maintained? So far, research shows that all cells have sophisticated molecular machinery to produce unusual bases in tRNAs and certain other RNAs. Some enzymes recognize a particular type of tRNA, excise the base from a specific nucleotide position, and replace it with another base; other enzymes chemically modify an existing base.
Modified bases seem to contribute in subtle ways to the thermodynamic stability of three-dimensionally structured RNAs such as tRNA and rRNA. For example, there are viable bacterial strains that differ only in their ability to modify rRNA at a specific site, due to the presence or absence of a particular rRNA-modifying enzyme. However, in a culture medium inoculated with equal amounts of the two strains, the strain containing the modifying enzyme eventually takes over the culture. This result implies that the modification of rRNA contributes to the efficient function of ribosomes, despite requiring extra cellular energy input.
Ribozyme Form Explains Function
Michel, F., M. Hanna, R. Green, D.P. Bartel, and J.W. Szostak. 1989. The guanosine binding site of the Tetrahymena ribozyme. Nature 342:391–395.
Stahley, M.R., and S.A. Strobel. 2006. RNA splicing: Group I intron crystal structures reveal the basis of splice site selection and metal ion catalysis. Curr. Opin. Struct. Biol. 16:319–326.
The discovery of ribozymes coincided with an important technological advance for molecular biologists: the ability to transcribe RNA molecules of any sequence in vitro and thereby test the function of RNAs in the complete absence of proteins. Furthermore, they could probe the molecular structure of ribozymes with chemicals that react with RNA nucleotides only when they are not involved in base-pairing interactions or packed against other nucleotides in the folded structure of the RNA.
An early observation was that mutations in the RNA sequence that disrupted parts of the three-dimensional structure also perturbed catalytic activity. For example, researchers noticed that a specific base pair in the Tetrahymena group I self-splicing intron was present in the same place in the secondary structure of all related group I introns. Changing this base pair to any other base combination disrupted the self-splicing reaction, because the intron was no longer capable of binding efficiently to GTP, a cofactor in the splicing reaction (Figure 3). In this way, investigators discovered that the Tetrahymena group I intron (and later, other ribozymes) has a defined three-dimensional shape that is essential to catalytic activity. Using in vitro transcribed and purified RNA, it was later possible to crystallize ribozymes and their component domains, revealing how these RNAs form active sites to enhance chemical reaction rates.
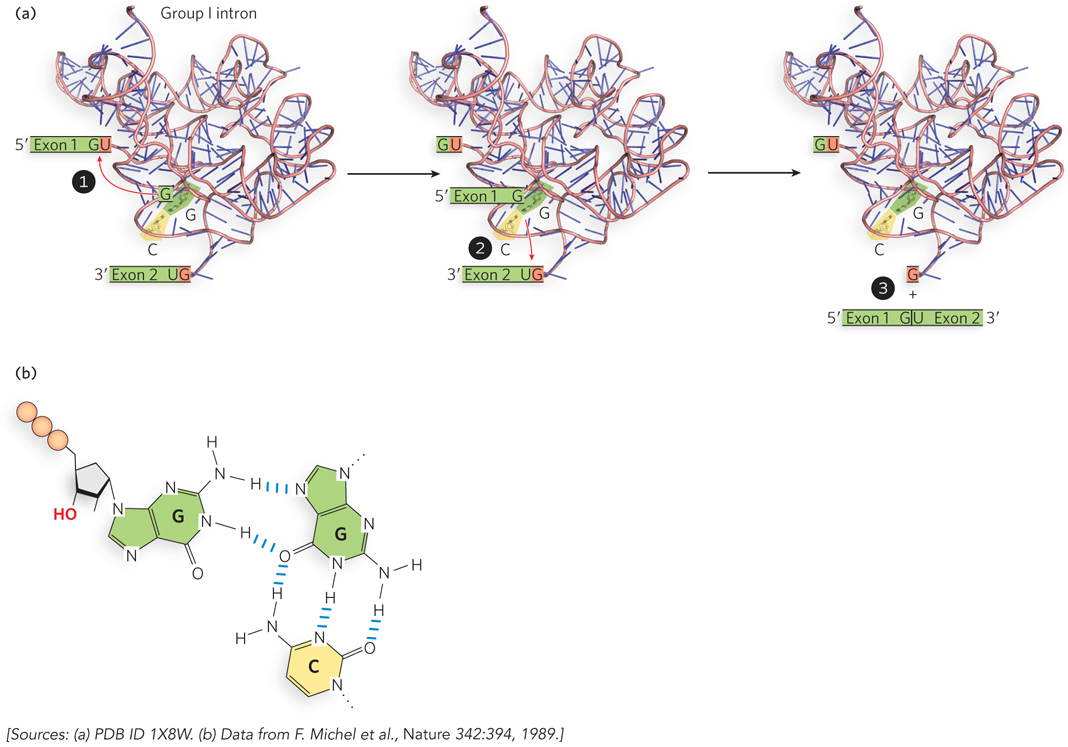
FIGURE 3 The group I intron structure includes a G≡C base pair that binds the GTP cofactor. (a) To initiate self-splicing, the group I intron structure precisely positions the G≡C base pair. (b) The G≡C binds a GTP cofactor through noncanonical base pairing.




