19.3 POSTTRANSCRIPTIONAL REGULATION OF GENE EXPRESSION
Thus far we have discussed the regulatory mechanisms involved in initiation of transcription, but as Figure 19-1 shows, regulatory mechanisms operate at many steps following transcription. Posttranscriptional processing of RNA and translation of mRNA into protein are regulated at several points. The regulation of protein synthesis at the initiation stage is common because, much like the strategy of regulating transcription at the initiation step, it saves the cell the huge energy investment of synthesizing the protein product. Nevertheless, there are some posttranslational regulatory mechanisms.
The regulation of gene expression after production of a functional protein does have several advantages. For example, it takes substantial time to produce mRNA and translate it into protein, so one benefit of regulating a pathway by acting on a fully formed protein is the speed with which changes in the amount or activity of the protein can be implemented. Covalent modification can turn a protein on or off very rapidly. Some types of gene regulation can be inherited through generations of cell division through imprinting (see Chapter 21) and epigenetics (see Chapter 10).
We explore here some of the main mechanisms of posttranscriptional regulation, occurring through mRNA processing and mRNA stability, translation initiation, covalent modification, cellular localization, and protein degradation.
Some Regulatory Mechanisms Act on the Nascent RNA Transcript
After transcription initiation, there are several ways in which a gene can be regulated before the mature mRNA transcript is produced. As an overview, we briefly describe three steps at which regulation can take place: transcript elongation, mRNA splicing, and modification of mRNA termini. These, and other examples, are discussed in more detail in Chapter 20 (for bacteria) and Chapter 22 (for eukaryotes).
Transcript Elongation One bacterial example of regulation affecting transcript elongation is a process known as attenuation. Attenuation prevents movement of the transcribing RNA polymerase into the first gene of an operon unless the proper conditions have been met. Controls to stop attenuation and thus proceed with transcription involve a delicate balance of metabolites, proteins, and mRNA structure. Attenuation is relatively common for the operons of amino acid biosynthesis and is particularly well documented for the trp operon of E. coli (see Chapter 20). In eukaryotes, many factors affect transcript elongation, and these elongation factors can be targets of control.
mRNA Splicing Many eukaryotic RNA transcripts contain introns that are spliced out in forming the mature mRNA (see Chapter 16). The splicing process is performed by a multiprotein spliceosome in the nucleus. Sometimes an mRNA has alternative splice junctions to choose from, which result in different products. The choice of splice site is regulated by repressors, activators, and enhancers in ways that seem to be mechanistically similar to the regulation of transcription initiation. Alternative splicing choice is thus another point at which gene expression can be regulated.
681
Modification of mRNA TerminiBoth the 5′ and 3′ ends of eukaryotic mRNAs are highly modified in multistep reactions (see Chapter 16). The 5′ terminus is modified by the addition of nucleotides connected by unusual phosphodiester bonds, referred to as the 5′ cap. The 3′ terminus is cleaved at a particular site prior to transcription termination, then multiple A residues are added to form a poly(A) tail. Specific proteins recognize and bind to these modifications, which are important in mRNA transport from the nucleus, mRNA stability in the cell, and efficient association of the mRNA with ribosomes and its use in translation. Exciting new discoveries are being made about control mechanisms at the level of these mRNA modification and transport steps.
Small RNAs Can Affect mRNA Stability
The amount of protein generated from a gene is dependent on the stability of the RNA message, and regulatory mechanisms have evolved to control mRNA stability. In higher eukaryotes, certain genes are “silenced” by a class of RNAs that interact with mRNAs, resulting in degradation of the mRNA or inhibition of translation. This form of gene regulation uses small RNAs. A variety of small RNAs can control developmental timing, repress the activity of transposons, or destroy invading RNA viruses, especially in plants, which lack an immune system. Small RNAs may also play a role in heterochromatin formation, which silences all the genes contained in the heterochromatin. The role of these small mRNAs in eukaryotic gene regulation is explored further in Chapter 22. Bacteria also contain a variety of small RNAs that act at several levels to regulate gene expression.
Small RNAs are sometimes called microRNAs (miRNAs). When present only temporarily, such as during development, transient small RNAs are referred to as small temporal RNAs (stRNAs). Hundreds of different miRNAs have been identified in more complex eukaryotes. They are transcribed as precursor RNAs, about 70 nucleotides long, that form hairpinlike structures (Figure 19-24a). An endonuclease trims the precursor RNAs to form short duplexes of 20 to 25 nucleotides, one strand of which anneals to the target mRNA. The best characterized of these endonucleases is Dicer; endonucleases in the Dicer family are widely distributed in eukaryotes.
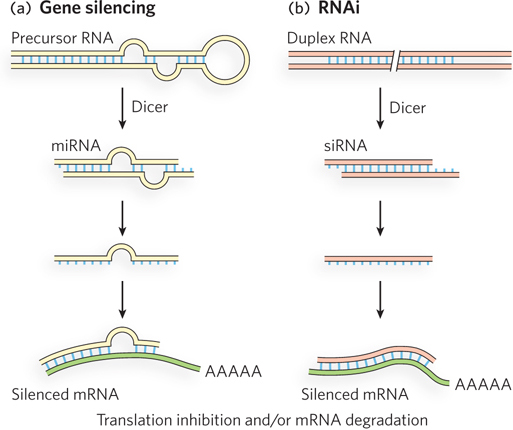
When the expression of genes producing miRNAs goes awry, tumors can result. As described in this chapter’s Moment of Discovery, overexpression of the miR-
Gene regulation mechanisms involving Dicer, besides their important physiological role, also have a very useful practical application. If an investigator introduces into an organism a synthetic duplex RNA corresponding to a target mRNA, Dicer cleaves the duplex into short segments called small interfering RNAs (siRNAs), which bind to and silence the mRNA (Figure 19-24b). This laboratory technique is called RNA interference (RNAi). In plants, almost any gene can be shut down in this way. In nematodes, simply feeding the duplex RNA to the worm silences the target gene. The technique is a very important tool in studies of gene function, because any gene can be silenced without constructing a mutant organism. The study of functional RNAs (such as miRNAs) is an exciting and relatively new area of molecular biology—
Some Genes Are Regulated at the Level of Translation
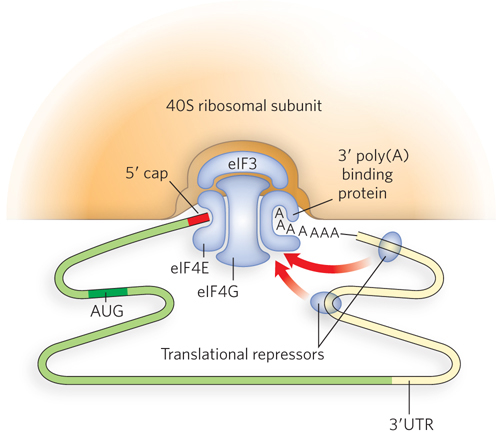
Some translational regulation does occur in bacteria, but it is much more common in eukaryotes because of the long half-
682
Global translational control also exists in bacteria and eukaryotes. For example, the ribosomal apparatus represents a large energy investment for the cell, and synthesis of the many components of the ribosome is regulated in processes linked to the cellular demand for proteins. Translational control of dozens of genes encoding ribosomal components is regulated by protein binding to the translation start sites in the mRNAs. Furthermore, if rRNAs are not present in sufficient amounts to match ribosomal protein subunits, the excess unassembled ribosomal proteins bind and inhibit translation of their respective mRNAs, forming a feedback translational control circuit.
Some Covalent Modifications Regulate Protein Function
Protein function can be dramatically altered by many types of covalent modification. Protein modifications include phosphorylation, acetylation, methylation, glycosylation, ubiquitination, and sumoylation (further discussed later in this section). The modifications can have various effects: they may render the protein active or inactive; result in a change in oligomeric state, with functional consequences; alter the protein’s affinity for DNA or for another protein; or affect the protein’s stability in the cell.
An example of proteins that are highly regulated by covalent modification is the subunits of nucleosomes. Recall from Chapter 10 that nucleosomal proteins have long N-
Modifications associated with the activation of transcription are recognized by enzymes that make the chromatin more accessible to the transcriptional machinery. When transcription of a gene is no longer required, certain modifications are enzymatically removed and others are added, marking the chromatin as transcriptionally inactive. The effect of histone modification on gene expression is discussed further in Chapter 21. Other examples of covalent modifications that direct gene expression are briefly described below and are expanded upon in Chapters 20–22.
Gene Expression Can Be Regulated by Intracellular Localization
In bacteria, transcription repressors and activators can undergo an allosteric change on binding a small effector molecule (such as allolactose or cAMP) that acts as a signal of environmental conditions, and in this way gene expression is repressed or activated in response to the signal (see Section 19.1). The compartmentation of eukaryotic cells affects the way in which gene expression can respond to environmental signals and provides opportunities for regulation at the level of transfer of proteins between intracellular locations. A prevalent pathway for communication with the extracellular environment in eukaryotes is through cell surface receptors. The receptors bind a signal molecule and relay its message through the plasma membrane by complex signal transduction pathways, eventually resulting in transcriptional regulation in the nucleus.
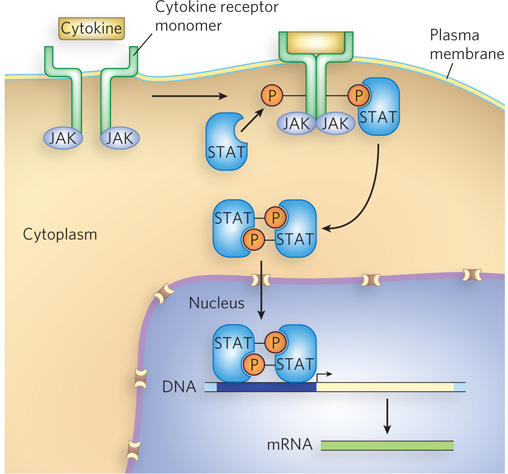
A relatively simple example of a signal transduction pathway is the JAK-
683
Dephosphorylation by specific protein phosphatases is also important in the regulation of transcription activator or repressor activity, and this sometimes involves cellular localization as a means of regulating gene expression—
Phosphorylation-
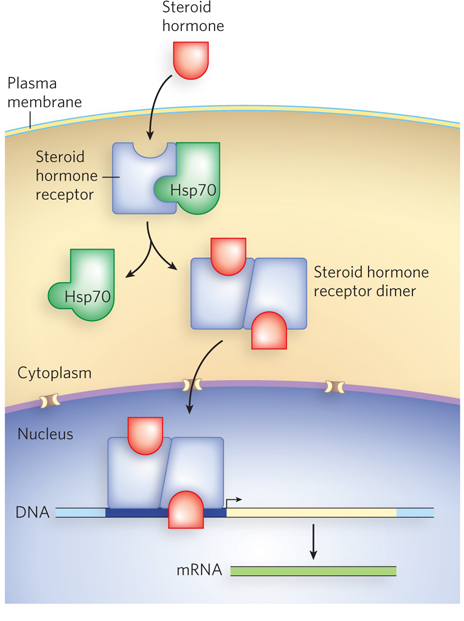
Steroid hormone receptors are another example of regulation by intracellular localization. These receptors are transcription activators, held in the cytoplasm by association with a heat shock protein, Hsp70. Steroid hormones are soluble in lipids and can pass through the plasma membrane without a specific transporter. On entry of the steroid hormone into a cell that expresses the particular steroid-
684
HIGHLIGHT 19-1 MEDICINE: Insulin Regulation: Control by Phosphorylation
Insulin is a small peptide hormone (51 residues), produced in the pancreas, that is central to the control of energy and glucose metabolism. For example, insulin stimulates glucose uptake from the blood by muscle, fat, and liver cells, and the use of glucose (in preference to fat) as an energy source. In these duties, insulin acts as a regulator of gene transcription.
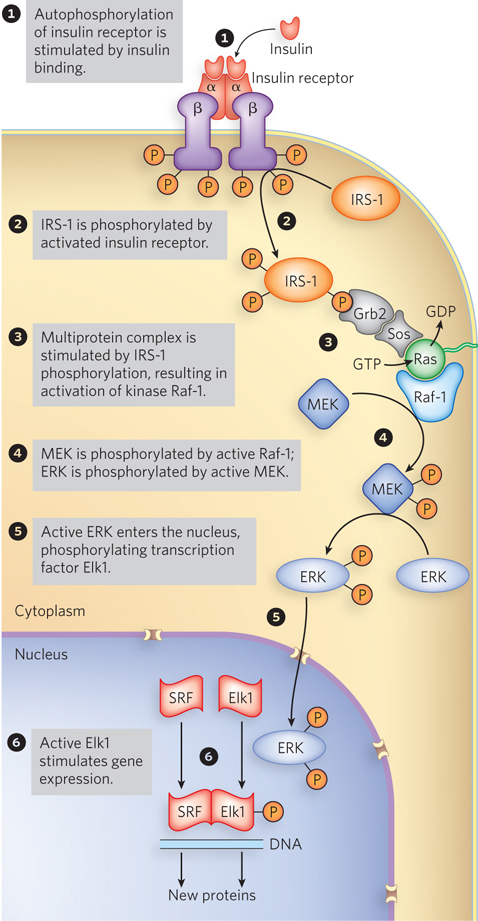
The insulin signaling pathway involves an extensive protein kinase cascade, resulting in activation of more than 100 genes. The signaling cascade is initiated when insulin binds to the membrane-
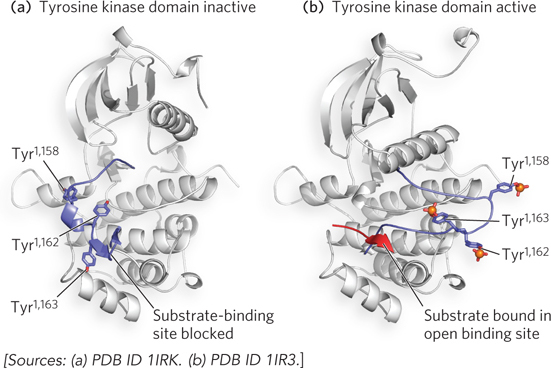
One of the target proteins in the insulin protein kinase cascade is insulin receptor substrate-
685
Insulin and the insulin receptor also regulate glycogen (a storage form of glucose) metabolism through another phosphorylation pathway that short-
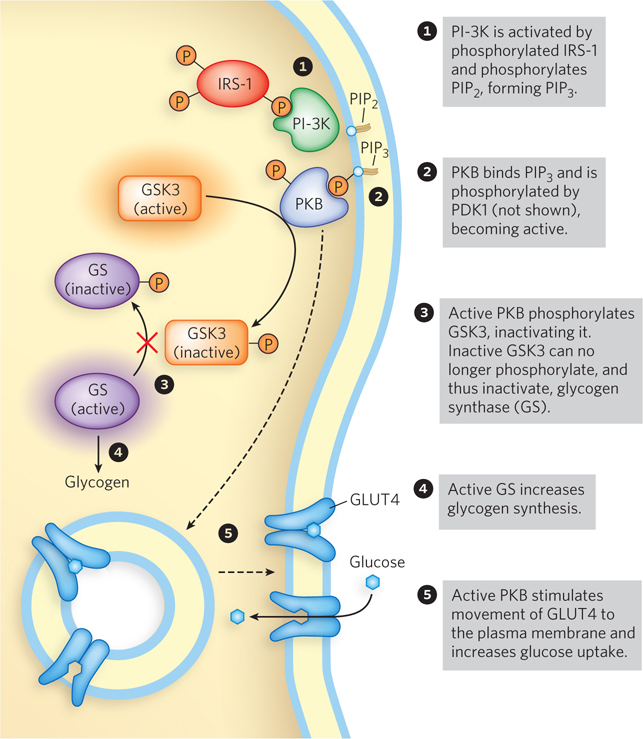
Glucose import into cells is yet another pathway controlled by insulin in a way that short-
686
The cell can also control the intracellular localization of a regulatory protein in the absence of signal transduction. Nuclear proteins, newly synthesized in the cytoplasm, contain a localization sequence that targets them to the nucleus. Cellular localization of a regulatory protein can thus be achieved by masking or unmasking the nuclear localization sequence, controlling access of the protein to the nucleus. Cells also regulate the localization of some proteins through covalent modification by the 101-
Protein Degradation by Ubiquitination Modulates Gene Expression
Once a protein has been produced in response to an environmental signal, it is important that the protein can be removed when it is no longer needed. Cells have a regulated mechanism for targeting proteins for removal through a protein degradation pathway. An efficient mechanism for proteolysis is also important for the turnover of misfolded or unfolded proteins, enabling recycling of their amino acids for the synthesis of new proteins. For protein removal, both bacteria and eukaryotes use a large, multisubunit, barrel-
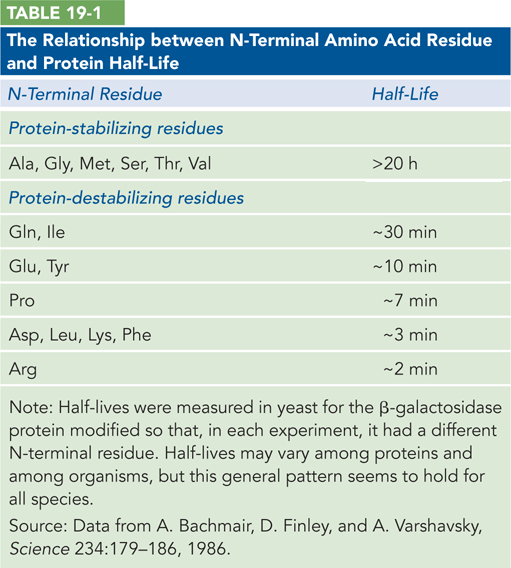
Although we do not yet understand all the signals that trigger recognition of a protein for degradation, one simple signal has been found. For many proteins, the identity of the first amino acid residue—
In eukaryotes, but not bacteria, regulated protein degradation is directed by the attachment of the 76-
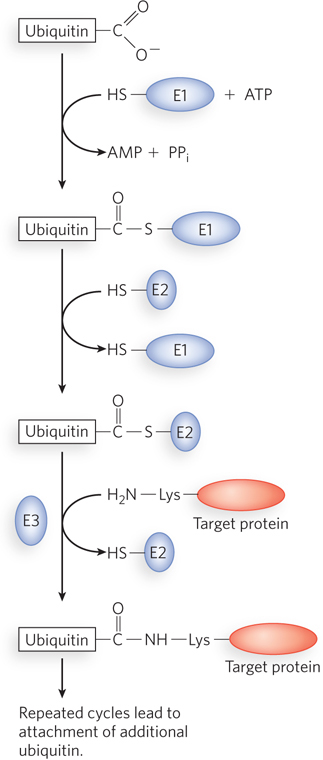
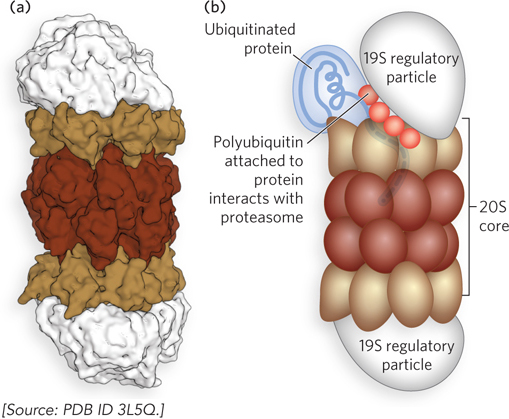
Ubiquitinated proteins are degraded by the 26S proteasome (Mr 2.5 × 106), shown in Figure 19-29. The proteasome consists of two copies each of at least 32 different subunits, which assort into two main subcomplexes: a barrel-
Not surprisingly, defects in the ubiquitination pathway have been implicated in a wide range of disease states. The inability to degrade certain proteins that activate cell division can lead to tumor formation, and the too rapid degradation of proteins that act as tumor suppressors can have the same effect. The ineffective or overly rapid degradation of cellular proteins also appears to play a role in a range of other conditions: renal diseases, asthma, neurodegenerative disorders (e.g., Alzheimer disease, Parkinson disease), cystic fibrosis (sometimes caused by overly rapid degradation of a chloride ion channel), and Liddle syndrome (in which a sodium channel in the kidney is not degraded, leading to excessive Na+ absorption and early-
687
Bacteria and eukaryotic organelles that evolved from bacteria also have proteasome-
SECTION 19.3 SUMMARY
Gene regulation can occur at various steps after transcription initiation. Points of regulation involving the RNA transcript include transcript elongation, splicing, modification, and stability. The stability of mRNAs can be affected by microRNAs.
Control of gene expression can occur at the level of translation initiation or elongation. Eukaryotes are particularly adept at regulating the initiation step.
Gene expression is also controlled at the level of protein products by several types of covalent modification, such as phosphorylation, acetylation, and methylation. Covalent modification carries the advantage of rapidly altering protein activity without waiting for changes in transcription and translation.
Protein targeting to particular intracellular compartments is another mechanism of gene regulation. Transcription factors can be excluded from the nucleus by phosphorylation or by binding of a regulatory protein that masks a nuclear localization signal. With degradation or modification of the regulatory protein, the transcription factor can enter the nucleus.
Gene expression can be regulated at the level of protein stability, which typically involves degradation by protease machinery. In eukaryotes, ubiquitination is used to direct proteins to the proteasome complex for degradation.
688
UNANSWERED QUESTIONS
The many levels of gene regulation required in cellular function and adaptation to changing conditions are coming into focus for molecular biologists. But the extra levels and structural complexities required for the development of multicellular organisms such as humans, with 50 trillion cells, still defy the imagination. As sophisticated as our current knowledge is, when we look back some years from now, it will probably appear quite primitive.
How extensive are the roles of microRNAs? New miRNAs are being found frequently. They function in various ways, but the details are still scarce and the diversity of functional mechanisms is only now becoming apparent. Some miRNAs are clearly implicated in cancer, making the understanding of these small regulatory molecules extremely important to human health.
How often is intracellular localization used to regulate protein function? Regulatory mechanisms at steps other than transcription, such as intracellular localization, are being discovered at a rapid pace and are proving to be of great importance to cellular function. Modifications that lead to compartmentalization of a protein can be quickly implemented, enabling rapid changes in the cell, and just as rapidly reversed, conserving the protein for repeated use. Because proteins and mRNAs are neither formed nor lost in this form of regulation, it provides a fuel-
efficient regulatory mechanism that may have more widespread use than is currently appreciated. How do regulatory mechanisms function together in the cell or whole organism? Our understanding of regulatory mechanisms for individual genes, and sometimes for several genes in a specific pathway, is growing. But it seems likely that for a cell to function efficiently in a complex environment, it must be capable of integrating sensory inputs of many sorts. We could hypothesize that different regulatory mechanisms engage in cross-
talk, possibly resulting in vast regulatory networks. We currently know little about how different regulatory paths communicate or interconnect in the cell. Further improvements in genomic techniques for systems biology, as well as increased computational power to categorize and analyze the data, are likely to have a huge impact on our understanding of how whole networks of interrelated proteins are regulated during cellular function and the development of complex organisms.
689
HOW WE KNOW: Plasmids Have the Answer to Enhancer Action
Dunaway, M., and P. Dröge. 1989. Transactivation of the Xenopus rRNA gene promoter by its enhancer. Nature 341:657–
During early studies of enhancer action, two major models were proposed for how enhancer-
The researchers placed an enhancer on one plasmid and a promoter on another plasmid, then topologically linked the plasmids together. They transferred these linked plasmids into Xenopus oocytes, along with a control plasmid containing the same promoter but no enhancer. If the enhancer-
To quantify transcription from the topologically linked plasmid versus the control plasmid, Dunaway and Dröge divided the lysate and performed S1 analysis using either a 40-
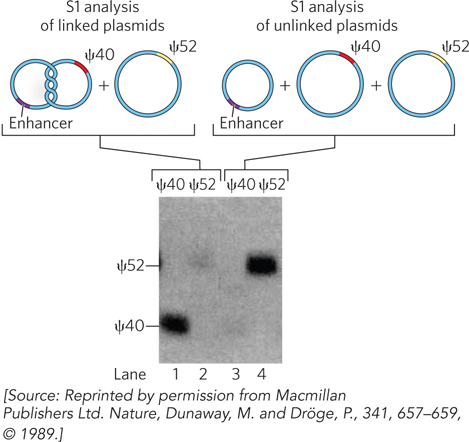
690