22.1 POSTTRANSCRIPTIONAL CONTROL INSIDE THE NUCLEUS
The multitude of posttranscriptional regulatory mechanisms in eukaryotes is usefully divided into those that occur in the nucleus and those that occur in the cytoplasm. In the nucleus, mRNA is modified in many ways and prepared for transport to the cytoplasm. In the cytoplasm, the focus shifts to translation. Control mechanisms determine which transcripts are translated, which are stored (and where they are stored), and how long each transcript is present in the cell. Additional controls are layered onto the translation process itself and the fate of the proteins thus produced (see Chapter 18).
Experiments to test which step in gene expression—transcription or translation—is regulated in cells often take advantage of molecular inhibitors that are specific for one step or the other. Two natural antibiotics made by bacteria of the genus Streptomyces have been particularly useful. Actinomycin D is a polypeptide that specifically blocks eukaryotic gene transcription by binding to DNA within the transcription initiation complex, preventing transcript elongation by RNA polymerase II (Figure 22-1a). Cycloheximide is a small molecule that interferes with the movement of tRNAs during polypeptide elongation on the ribosome, thus blocking protein synthesis (Figure 22-1b). These compounds are useful tools for the preliminary dissection of mechanisms of eukaryotic gene regulation. For example, Mark Ashe and Alan Sachs discovered that yeast cells that are starved for sugar rapidly shut down their protein synthesis. To determine whether this regulation is based in transcription or translation, yeast cells growing in a nutrient-rich medium were isolated by centrifugation and transferred to a medium containing no sugar. Protein production in these cells, as measured by the abundance of large ribosome-mRNA complexes, decreased to almost zero within a few minutes. On addition of sugar to the growth medium, protein synthesis was restored. Actinomycin D had no effect on either the inhibition or the reactivation of protein production, showing that no new transcription was needed for this kind of regulation.
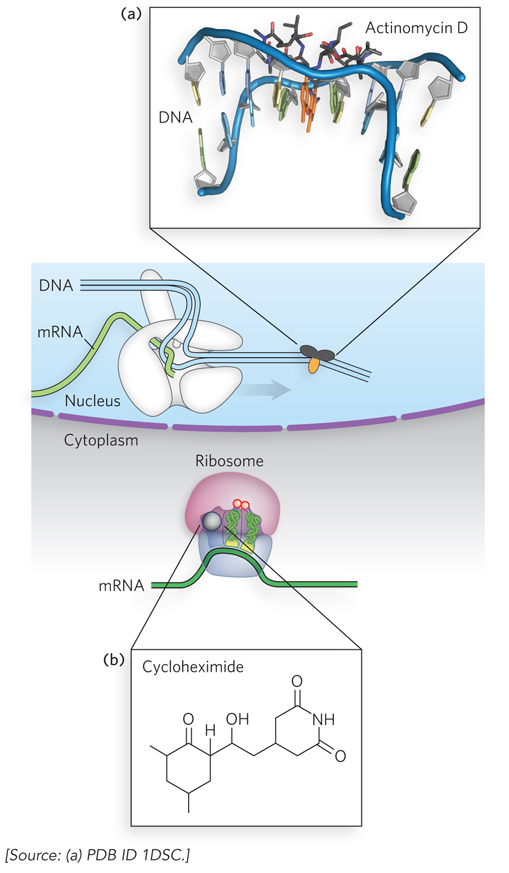
Figure 22-1: Compounds that selectively block transcription or translation. (a) Actinomycin D blocks transcription elongation by RNA polymerase II, by inserting itself into DNA. (See Figure 15-8 for the chemical structure of actinomycin D.) (b) Cycloheximide blocks translation elongation by the ribosome.
In this section, we embark on detailed descriptions of the processes by which mRNAs are altered in the nucleus to control the temporal expression and structure of the final gene products. Regulation by the alteration of mRNA sequences can allow a single gene to yield more than one protein product, thus optimizing the efficiency of information storage in a genome. We focus on some examples of mRNA alterations that illustrate this potential, including mRNA splicing, 3′-end cleavage, and mRNA transport. These processes were introduced in Chapter 16 and are expanded on here. RNA editing, another process that makes important contributions to the coding potential of some eukaryotic mRNAs, is also described in Chapter 16.
Alternative Splicing Controls Sex Determination in Fruit Flies
The splicing of a new mRNA transcript does not always lead to the exclusive excision of all the introns. Instead, for some genes, one or more exons may also be deleted during maturation of a subset of the mRNAs. This process, called alternative splicing, creates new forms of the mature transcript that encode versions of the protein lacking one or more peptide segments. In this way, two or more different proteins can be produced from a single coding sequence.
Alternative splicing has a substantial effect on the coding capacity of eukaryotic genomes. It is relatively rare in single-celled eukaryotes such as yeast, but its frequency increases in multicellular organisms. Alternative splicing affects more than 95% of mammalian genes. Anywhere from a few to several thousand different mRNA variants may be generated from a single gene. The existence of alternative splicing implies a level of complexity in the splicing process that goes beyond the signal sequences at the exon-intron boundaries and the spliceosome proteins that bind to them, as described in Chapter 16. The process relies on an extra layer of mRNA sequences and the trans-acting proteins that bind to them to determine which splicing events occur in which cells and under what conditions. That extra layer of control can also be inferred from an examination of introns themselves. Whereas most exons fall within a range of 100 to 300 nucleotides in length, introns exhibit much more variation, ranging from about 50 to more than 100,000 nucleotides. In longer introns, canonical splicing signals that could lead to removal of parts of the intron are common, but are ignored by the cellular splicing systems.
The extra layer of control that guides alternative splicing and silences inappropriate splicing events is provided by mRNA sequences in the exons and introns, called exonic splicing enhancers and exonic splicing silencers (ESEs and ESSs, respectively) and intronic splicing enhancers and intronic splicing silencers (ISEs and ISSs). Whether a sequence is an enhancer or a silencer depends a great deal on the proteins that bind to it. A wide range of trans-acting RNA-binding proteins have been discovered, each one recognizing one or more of these sequences and thereby affecting the splicing process. There are two main families of alternative splicing regulatory proteins, the Ser/Arg-rich (SR) proteins and the heterogeneous nuclear ribonucleoproteins (hnRNPs). Most of these proteins interact with components of the eukaryotic spliceosome. Their function is illustrated in Figure 22-2.
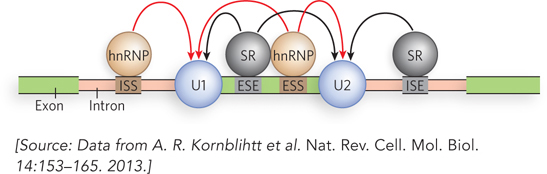
Figure 22-2: The regulation of splicing. Whether or not an exon is included in the final, mature mRNA transcript in a eukaryotic cell depends on a range of sequences in the primary transcript and the regulatory proteins that bind to them. The sequences that participate in splice site activation are exonic splicing enhancers (ESEs) or intronic splicing enhancers (ISEs), located in the exon or in the adjacent intron. The sequences that participate in splice site silencing are exonic splicing silencers (ESSs) and intronic splicing silencers (ISSs). The most common families of regulatory proteins that bind to these sequences are the serine/arginine-rich proteins (SR proteins) and the heterogeneous nuclear ribonucleoproteins (hnRNPs).
In Drosophila, an interesting example of alternative pre-mRNA splicing dictates sexual fate during development. It is a complex system, but not unusually so in the world of alternative splicing. In both male and female flies, each cell contains two copies of the autosomes; female cells also have two X chromosomes, and male cells contain just one X chromosome (unlike in mammals, an X chromosome is not inactivated in fruit flies). The resulting difference in the ratio of X chromosomes to autosomes leads to different levels of expression of the X-chromosomally encoded SisA (sisterless) and SisB transcription activators. These regulatory proteins, along with an autosomally encoded repressor called Deadpan (Dpn), act on the Sex-lethal (Sxl) gene. The Sxl protein is an RNA-binding protein that regulates splicing. In females, with SisA and SisB in twice the amounts found in males, Sxl is activated; in males, Sxl is repressed.
Two promoters govern Sxl expression: Pe (establishment) and Pm (maintenance) (Figure 22-3a). The Sxl gene has one exon, L3, that contains an in-reading-frame UGA stop codon, and this exon must be removed if a full-length protein is to be produced. When Sxl is expressed from Pe, the primary transcript does not include the L1, L2, or L3 exons. Due to the action of regulatory components (e.g., ESS and ESE sites) not yet identified, the default splicing pathway of this transcript connects the exon E1 (encoding the N-terminal 20 amino acid residues of the protein) directly to L4. The splice junctions at the ends of exons L2 and L3 are not recognized by the splicing machinery and thus are deleted along with the neighboring introns. The mature transcript encodes an active Sxl protein with the N-terminal residues encoded by exon E1. When Sxl is expressed from Pm, the default splicing pathway eliminates all introns precisely; translation is initiated in L2 and halted at the UGA stop codon in L3, and inactive Sxl protein results.
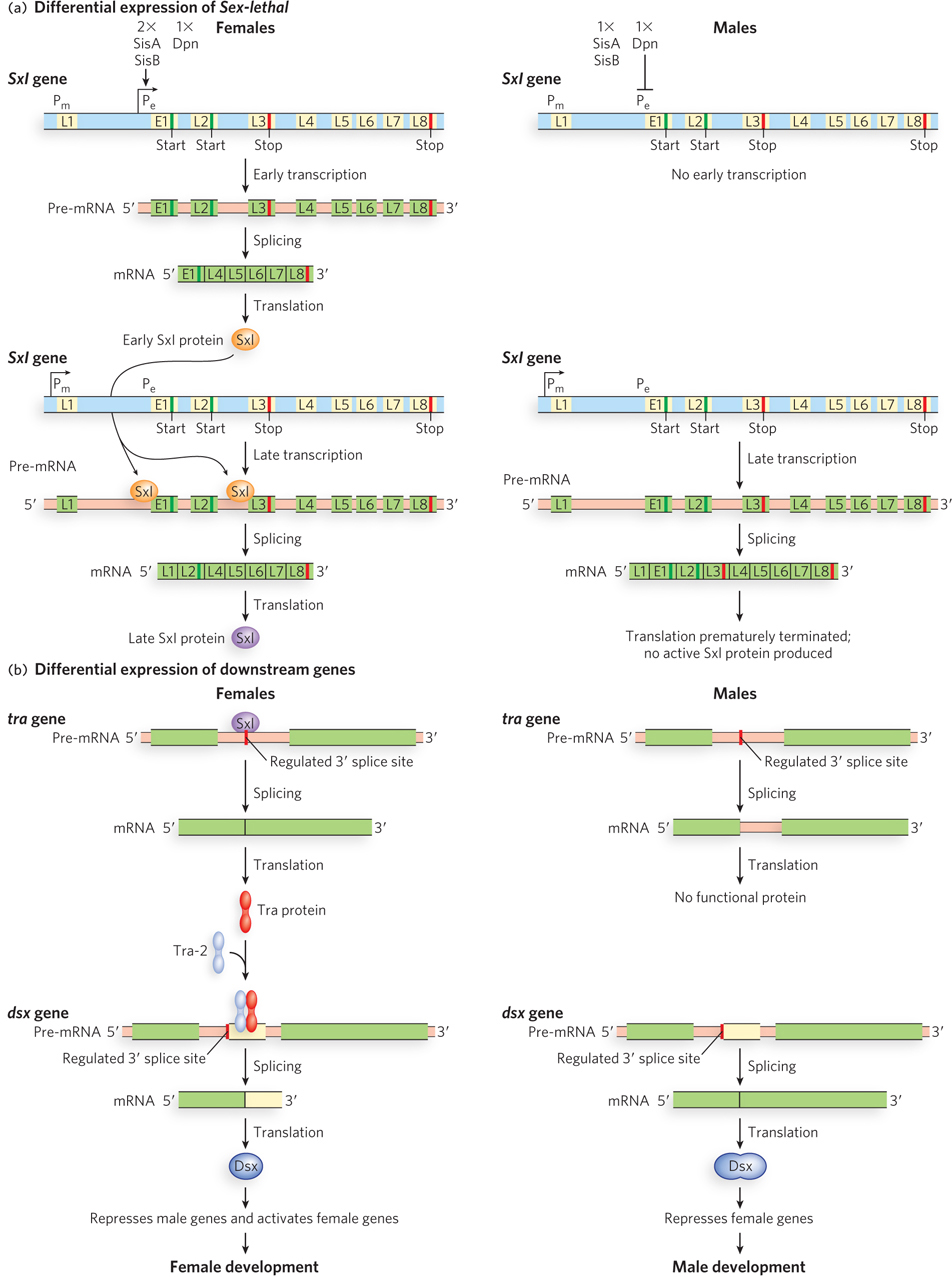
Figure 22-3: Alternative splicing of Sxl mRNA in male and female fruit flies. (a) The structure of the Sxl gene, showing the exons, introns, promoters, start codons, and stop codons, and its splicing patterns. In females, transcription from promoter Pe results in a characteristic splicing pattern and the production of a burst of active Sxl protein (top left). Later transcription from Pm, in the presence of Sxl, continues to produce active Sxl protein (bottom left). In males, transcription from Pe is repressed (top right). The absence of Sxl leads to a different splicing pattern of Pm transcripts and premature termination of Sxl (bottom right). (b) Sxl protein mediates differential expression of genes further downstream in the developmental pathway. In females, active Sxl protein produced from Pm leads to production of proteins that facilitate female development—first Tra protein, which then, with Tra-2, facilitates splicing of the mRNA for active Dsx protein (left). In males, the absence of Sxl leads to a different splicing pattern of tra transcripts; a different version of Dsx is produced that leads to male development (right).
In females, higher levels of SisA and SisB overcome Dpn repression and activate Pe early in development, generating active Sxl protein. Pm is switched on slightly later, and transcription from Pm is not dependent on SisA, SisB, or Dpn. The Sxl protein, already present at this point in development, is a splicing repressor that binds to the primary Pm-dependent transcript at particular ISSs and prevents the splicing machinery from recognizing the splice junctions on either side of the E1 or L3 exon. L1 is spliced directly to L2, and L2 to L4. In this way, the L3 exon is spliced out of the transcript as part of a larger segment effectively containing two introns, and production of functional Sxl protein continues (now with N-terminal residues encoded by L2).
The Sxl protein does not simply facilitate its own expression, however; it is also needed to regulate the expression of several additional genes downstream in the female developmental pathway (Figure 22-3b, left). Sxl regulates the splicing of additional transcripts, including that from the transformer (tra) gene. Sxl-mediated alternative splicing produces transcripts that encode functional Tra protein, another RNA-binding protein that regulates splicing. The Tra protein activates splicing of pre-mRNA from the double sex (dsx) gene, such that the truncated protein expressed from this spliced form represses the expression of male-specific genes.
In males, Pe is repressed by Dpn and never activated, due to the lower levels of SisA and SisB than in females. There is no early production of the Sxl protein. When, later, the expression of Sxl occurs from Pm, the lack of presynthesized Sxl protein leads to splicing of the Pm-produced transcript via its default pathway, with production of inactive Sxl fragments (see Figure 22-3a). Without functional Sxl protein to regulate splicing of the tra gene, no functional Tra protein is produced. In the absence of Tra protein, splicing of the dsx transcript produces mRNAs encoding an extended form of Dsx protein that represses transcription of female-specific genes. In the absence of genes enforcing the female developmental pathway, a male-specific pathway is initiated (see Figure 22-3b, right).
This interwoven regulatory cascade, with many steps involving alternative splicing, provides exquisite control over the sexual development of fruit flies by ensuring that sex-specific transcription patterns are maintained.
Multiple mRNA Cleavage Sites Allow the Production of Multiple Proteins
The 3′ cleavage and polyadenylation of eukaryotic mRNAs is carried out by a protein complex recognizing a site defined, in part, by the sequence AAUAAA. This is a key event in the maturation of mRNAs (see Chapter 16). Many genes have multiple sites for 3′-end cleavage. By regulating which site is used, cells can produce different proteins from a single gene. Sequencing of the human genome has revealed that as many as 60% of the genes may have multiple alternative 3′-end cleavage sites. This represents another point of regulation and yet another mechanism whereby the coding capacity of a eukaryotic genome is increased.
A well-studied example is the gene encoding immunoglobulin M (IgM) heavy chains. As the B cells (B lymphocytes) of the immune system mature, they enter a quiescent state in which each cell expresses a unique IgM on its surface. The many different B cells express immunoglobulins with different binding specificities, enabling the immune system to respond to a wide array of antigens. The membrane-bound IgM is generated by a splicing event in the primary transcript that eliminates one of two 3′-end cleavage sites and attaches two exons (M exons) that encode a series of hydrophobic amino acid residues at the C-terminus of the IgM; this hydrophobic sequence serves to anchor the protein in the membrane (Figure 22-4). When an antigen appears in the extracellular environment that is recognized by the IgM of a particular B cell, this triggers cellular signaling that leads to rapid proliferation (sometimes called clonal expansion) of that B cell. As the proliferation proceeds, a change occurs in the processing of the IgM mRNAs. An increased concentration of the protein CstF-64 (cleavage stimulation factor) leads to predominant use of the first 3′-end cleavage site of the IgM transcript rather than its deletion by splicing. The resulting IgM molecules no longer have the membrane anchor and are secreted into the surrounding plasma to help neutralize the threat posed by the antigen.
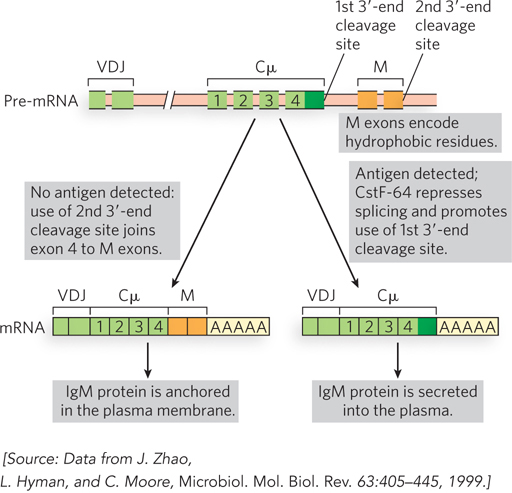
Figure 22-4: Alternative 3′-end cleavage sites and the fate of IgM proteins. In the absence of antigen (left), the first cleavage site is spliced out during mRNA processing, leaving the second site and the two M exons encoding hydrophobic C-terminal sequences that anchor the IgM in the membrane. When the cell encounters antigen (right), the splicing reaction that eliminates the first cleavage site is suppressed through the action of CstF-64; the mature mRNA produces an IgM that is secreted. Cμ exons encode the constant region of the IgM heavy chain. VDJ exons encode the variable, diversity, and joining segments of the IgM heavy chain.
Nuclear Transport Regulates Which mRNAs Are Selected for Translation
Incompletely processed mRNAs, such as those that still contain introns, are not exported from the nucleus and are degraded by the nuclear exosome. If this mechanism could be suppressed, various mRNAs made from the same gene, some containing one or more introns, could be exported from the nucleus. Translation would then produce different proteins from these mRNAs.
There is a regulatory mechanism that overcomes the nuclear surveillance of introns in mRNA, and it is used strategically by the human immunodeficiency virus. HIV makes one very long pre-mRNA, which is spliced in a variety of ways to make more than 30 mature mRNAs. Some of these mRNAs contain introns that are spliced out in other mRNAs but become part of the coding sequence in those where they are retained. These intron-containing mRNAs must be exported from the nucleus to be translated, and that export would normally be blocked by the splicing signals in the introns. To subvert the cell’s nuclear surveillance process, HIV encodes a protein called Rev. After synthesis in the cytoplasm, Rev is transported into the nucleus and binds to introns in HIV transcripts (Figure 22-5). Rev also binds transport receptors, and thus escorts intron-containing viral RNAs out of the nucleus.
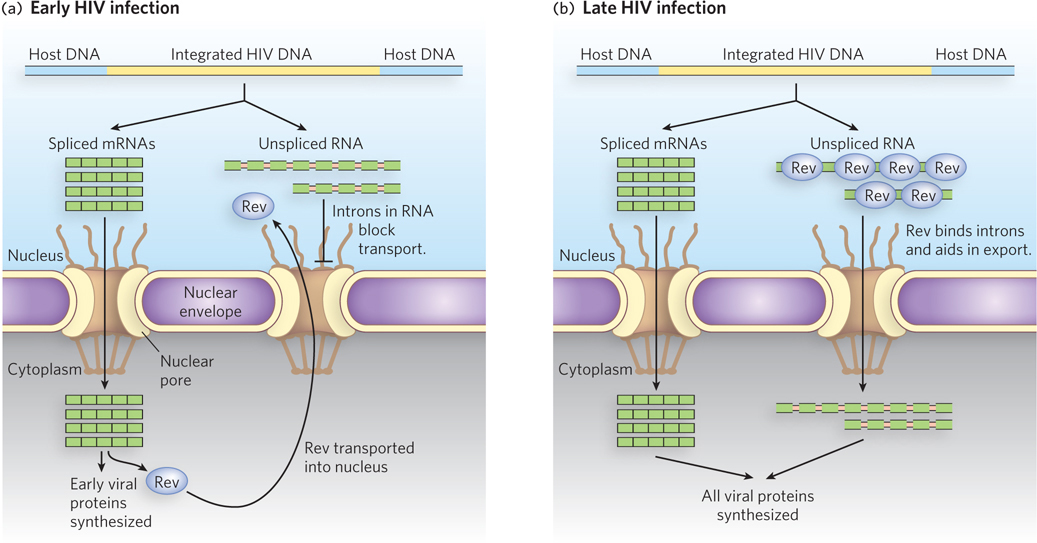
Figure 22-5: Regulation of nuclear export by the HIV Rev protein. (a) Early in HIV infection, only fully spliced viral mRNAs, containing the coding sequences for Rev and other proteins, are exported from the host cell nucleus and translated. (b) Once sufficient Rev protein has accumulated and been transported into the nucleus, unspliced viral RNAs can be exported into the cytoplasm. Many of these RNAs are translated into proteins. These and all other viral proteins are packaged into new viral particles.
SECTION 22.1 SUMMARY
Eukaryotic cells, and the cells of multicellular organisms in particular, express just a subset of proteins, depending on cell type and in response to various chemical signals. To maximize versatility, even at the expense of producing unneeded transcripts, cells sometimes regulate gene expression posttranscriptionally.
Two natural antibiotics, actinomycin D and cycloheximide, block eukaryotic transcription and translation, respectively. These are useful tools for determining which step of gene expression is regulated in response to a particular stimulus.
Alternative splicing greatly increases the coding capacity of eukaryotic genomes and is especially important in mammals.
Alternative splicing is influenced by splicing enhancer and splicing silencing sites located in both exons and introns.
In fruit flies, alternative splicing of the pre-mRNA encoding the Sex-lethal (Sxl) protein determines sexual fate during development.
Alternative selection of sites for 3′-end cleavage determines the fate of IgM molecules produced by B cells.
HIV produces a protein (Rev) that suppresses cellular intron surveillance and allows export of intron-containing viral transcripts from the nucleus.




