2.2 Temperature and Heat
Understand the difference between temperature and heat.
The human body’s perception of air temperature and the actual measured air temperature are not the same. A cold day feels colder when it is windy, creating the wind chill temperature. A hot day feels hotter when it is humid, creating the heat-
heat-index temperature
The temperature perceived by people as a result of high atmospheric humidity coupled with high air temperatures.
What Is Temperature?
So what exactly is temperature, and how do we measure it objectively? Temperature is defined as the average speed of molecular movement (or level of excitation) within a substance or object, and it is measured with a thermometer. Molecular movement is a form of kinetic energy. (Matter is made of molecules and atoms, but the term molecules will be used in this discussion as a generalization.)
temperature
The average kinetic movement of atoms and molecules of a substance.
Molecules move quickly in objects with high temperatures and relatively slowly in objects with low temperatures. If we were to somehow lower the temperature of an object to the point at which the molecules no longer moved, we would reach a point of 0 kelvins, or absolute zero. The Kelvin scale was developed by the British scientist Lord Kelvin (1824–
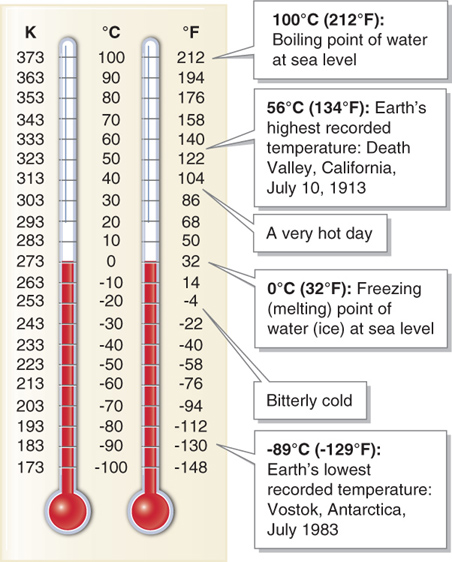
In the early 1700s, the German scientist Daniel Gabriel Fahrenheit developed the Fahrenheit scale (abbreviated F). A few years afterward, the Swedish astronomer Anders Celsius introduced the Celsius scale (abbreviated C). Water freezes at 0°C (32°F) and boils at 100°C (212°F). Most countries use the Celsius scale, which is part of the metric system of weights and measures (see Section GT.1). The public in the United States is generally most familiar with the U.S. customary system, which uses the Fahrenheit scale. Students in the United States will therefore find it helpful to be able to convert the temperature scales back and forth. Each Celsius degree is 1.8 times larger than a Fahrenheit degree. A formula for converting °C to °F is
°F = (1.8 × °C) + 32
A formula for converting °F to °C is
°C = (°F − 32)/1.8
The Crunch the Numbers feature provides conversion practice.
CRUNCH THE NUMBERS: Temperature Conversions
CRUNCH THE NUMBERS: Temperature Conversions
Convert the following temperatures. (Round to the nearest whole number.)
Celsius to Fahrenheit (use a calculator):
Question 2.2
30°C = _____ °F
Question 2.3
20°C = _____ °F
To quickly estimate the conversion from Celsius to Fahrenheit: Double and add 30.
Celsius to Fahrenheit (without a calculator):
Question 2.4
30°C = _____ °F
Question 2.5
20°C = _____ °F
Convert the following temperatures:
Fahrenheit to Celsius (use a calculator):
Question 2.6
90°F = _____ °C
Question 2.7
70°F = _____ °C
To quickly estimate the conversion from Fahrenheit to Celsius: Subtract 30 and divide by 2.
Fahrenheit to Celsius (without a calculator):
Question 2.8
90°F = _____ °C
Question 2.9
70°F = _____ °C
What Is Heat?
Temperature and heat are not the same. Temperature, as we have seen, is the average speed of molecular movement in a substance. Heat is the internal energy transferred between materials or systems due to their temperature differences. Molecules are too small to see, but the kinetic energy of their movement can be felt as heat. With a temperature increase, objects feel hotter because heat flows from objects of high temperature to objects of low temperature.
heat
The internal energy transferred between materials or systems due to their temperature differences.
To illustrate this, let’s imagine two identical ceramic mugs half full of hot coffee of the same temperature. Because each has the same temperature and the same amount of coffee, each has the same amount of internal energy.
Now imagine pouring all the coffee from one mug into the other so that one mug is full and the other is empty. The temperature of the full mug of coffee did not change, but its amount of internal energy doubled because it now has twice as much mass (or coffee).
Now imagine carefully setting the mug of hot coffee in a bowl of ice water, making sure no water spills into the coffee. Heat will flow out directly from the surface of the coffee into the surrounding air. The heat will also move out of the cup of coffee into the surrounding water and warm that water, and the coffee’s temperature will decrease (Figure 2.13).
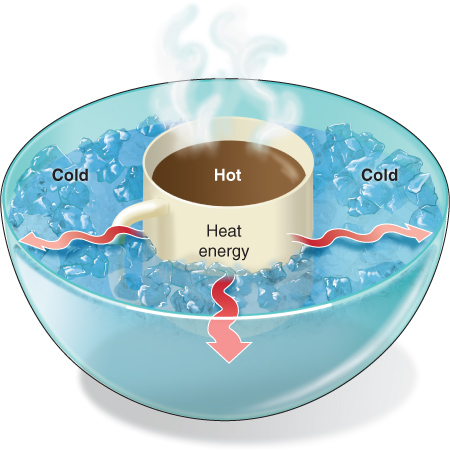
How Heat Moves
Heat is transferred through space and Earth’s physical systems in three ways: by conduction, convection, and radiation.
Conduction is the process by which energy is transferred through a substance or between objects in direct contact. When the coffee was poured into the ceramic mug, the temperature of the mug increased as the coffee’s heat was transferred to the molecules in the mug. In conduction, heat always flows from objects of higher temperature to objects of lower temperature. Heat transfers faster when the temperature difference is greater and when the material through which it is traveling is a good conductor of heat (Table 2.1). Metals are good heat conductors, but still air is not. Air is an insulator (any material that inhibits energy from transferring through it). That is why warm jackets and the fur of animals in cold climates are thickly padded with air.
conduction
The process by which energy is transferred through a substance or between objects in direct contact.
|
MATERIAL |
HEAT CONDUCTIVITY* |
|---|---|
|
Copper |
0.99 |
|
Glass |
0.0025 |
|
Dry sand |
0.0013 |
|
Water at 20°C |
0.0014 |
|
Wool felt |
0.0001 |
|
Air at 0°C |
0.000057 |
|
* Heat conductivity is defined as the quantity of heat in calories (c) that would flow through a square centimeter of the above materials in 1 second, given a temperature gradient of 1°C per centimeter |
|
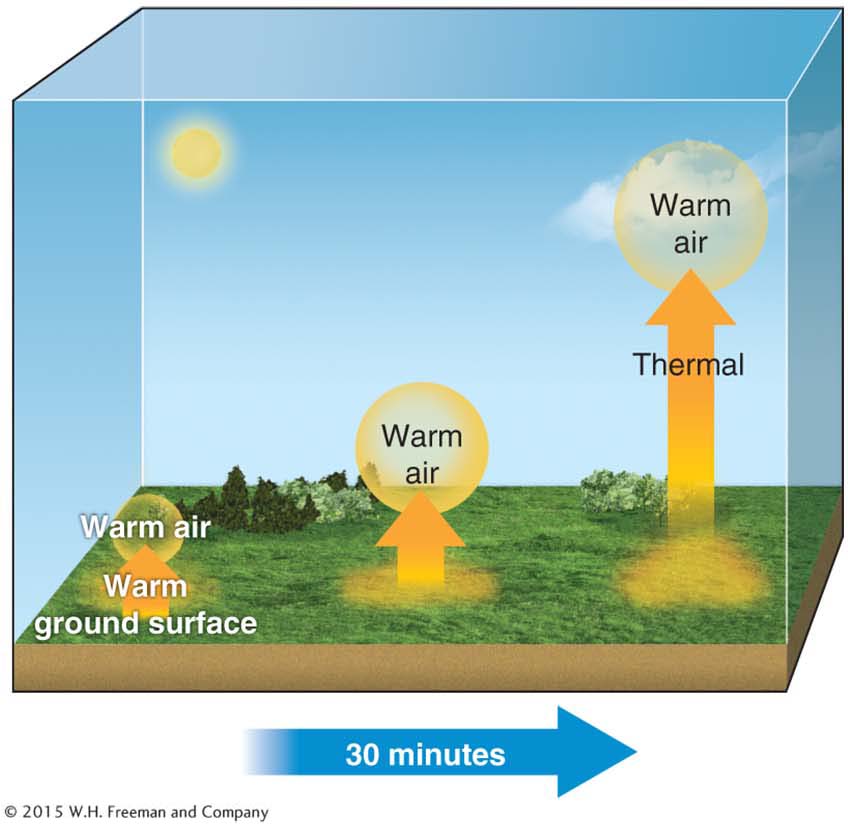
Convection is the transfer of heat through movement of mass within a fluid (a gas or liquid). Gases and liquids move and flow easily in response to differences in heating. For example, when the ground is warmed by sunlight, it heats the air above it. This heated warm air expands and becomes less dense, then rises, as shown in Figure 2.14.
convection
The transfer of heat through movement of mass within a fluid (liquid or gas).
Radiation is the process by which wave energy travels through the vacuum of space or through a physical medium such as air or water. Sunlight is an example of radiation. Radiation and electromagnetic energy are discussed in more detail in Section 2.4.
radiation
The process by which wave energy travels through the vacuum of space or through a physical medium such as air or water.