5.1 Neurons: The Building Blocks of the Brain
The brain may look inactive to the naked eye, but at a microscopic level it is the most dynamic organ of the body. It makes up about 2 percent of an average person’s body weight, but consumes about 20 percent of the person’s metabolic energy (Magistretti, 2008). The brain is an even more expensive organ to run during early childhood, with the brain metabolism of 4- and 5-year-old children being about 150% of adults’, falling to adult levels at about age 9 (Chugani et al., 1987). It is by far the most complex and compact computing machine in the known universe. The human brain contains roughly 80 to 100 billion nerve cells, or neurons, and roughly 100 trillion points of communication, or synapses, between neurons. These are all more-or-less constantly active, and their collective activity monitors our internal and external environments, creates all of our mental experiences, and controls all of our behavior. Figure 5.1 provides three different views of the adult brain.
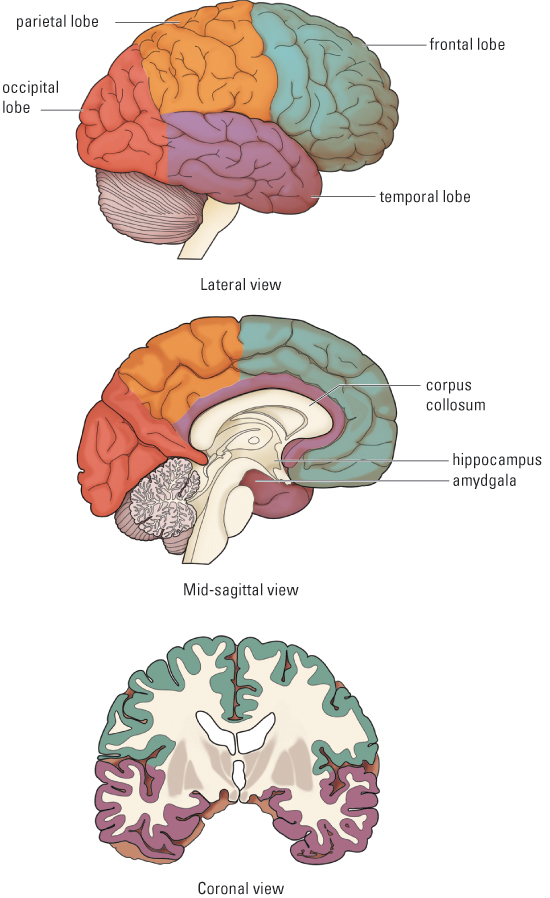

The seeming magic of the nervous system—its abilities to analyze sensory data, create mental experiences, and control movements in adaptive ways—lies not in the individual neurons, but in the organization of their multitudes. Yet each neuron is itself a complex decision-making machine. Each neuron receives information from multiple sources, integrates that information, and sends its response out to many other neurons or, in some cases, to muscle cells or glands. In this section we examine the structure and functions of individual neurons as a prelude to examining the larger structures and functions of the nervous system as a whole.
Three Basic Varieties of Neurons, and Structures Common to Them
The overall layout of the human nervous system is sketched in Figure 5.2. The brain and spinal cord (which extends from the brain down through the bones of the spinal column) make up the central nervous system. Extensions from the central nervous system, called nerves, make up the peripheral nervous system.
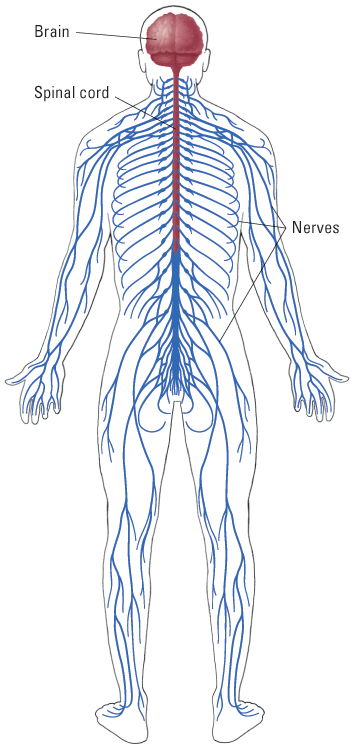
Don’t confuse the terms neuron and nerve. A neuron, as just defined, is a single cell of the nervous system. A nerve is a bundle of many neurons—or, more precisely, a bundle consisting of the axons (defined below) of many neurons—within the peripheral nervous system. Nerves connect the central nervous system to the body’s sensory organs, muscles, and glands. Note also that despite their names, the central and peripheral nervous systems are not two separate systems, but are parts of an integrated whole.
1
What are three types of neurons, and what is the function of each?
Neurons come in a wide variety of shapes and sizes and serve countless specific functions. At the broadest level of analysis, they can be grouped into three categories according to their functions and their locations in the overall layout of the nervous system (see Figure 5.3):
- Sensory neurons, bundled together to form nerves, carry information from sensory organs (including the eyes, ears, nose, tongue, and skin) into the central nervous system.
- Motor neurons, also bundled into nerves, carry messages out from the central nervous system to operate muscles and glands.
- Interneurons exist entirely within the central nervous system and carry messages from one set of neurons to another. Interneurons collect, organize, and integrate messages from various sources. They vastly outnumber the other two types.
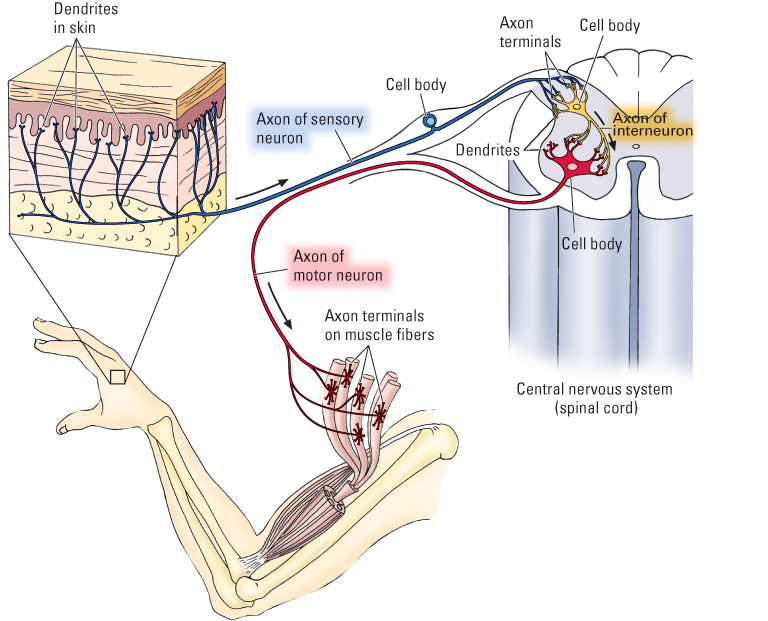
The human nervous system contains a few million sensory and motor neurons and roughly 100 billion interneurons. Our interneurons make sense of the input that comes from sensory neurons, generate all our mental experiences, and initiate and coordinate all our behavioral actions through their connections to motor neurons.
2
What are the main parts common to all or most neurons, and what is the function of each part?
Although neurons vary tremendously in shape and size, most contain the same basic parts. The parts, listed below, are labeled for all three types of neurons in Figure 5.3 and illustrated more fully for a motor neuron in Figure 5.4.
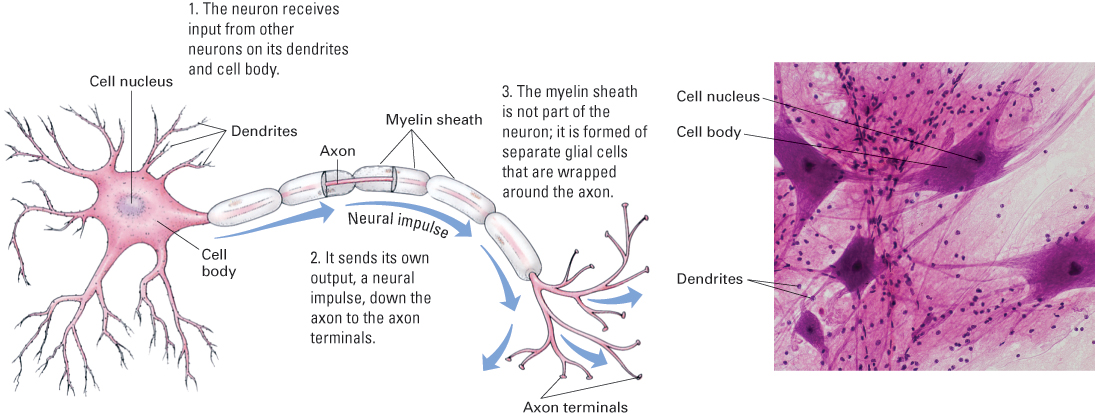
- The cell body is the widest part of the neuron. It contains the cell nucleus and other basic machinery common to all bodily cells.
- Dendrites are thin, tube-like extensions that branch extensively and function to receive input for the neuron. In motor neurons and interneurons, the dendrites extend directly off the cell body and generally branch extensively near the cell body, forming bush-like structures. These structures increase the surface area of the cell and thereby allow for receipt of signals from many other neurons. In sensory neurons, dendrites extend from one end of the axon, rather than directly from the cell body. They extend into a sensory organ and respond to sensory signals, such as sound waves in the ear or touch on the skin (shown for skin in Figure 5.3).
151
- The axon is another thin, tube-like extension from the cell body. Its function is to carry messages to other neurons or, in the case of motor neurons, to muscle cells. Although microscopically thin, some axons are extremely long. You have axons of sensory neurons extending all the way from your big toe into your spinal cord and then up to the base of your brain—a distance of 5 feet or more. Most axons form many branches some distance away from the cell body, and each branch ends with a small swelling called an axon terminal. Axon terminals are designed to release chemical transmitter molecules onto other neurons or, in the case of motor neurons, onto muscle cells or glandular cells. The axons of some neurons are surrounded by a casing called a myelin sheath.Myelin is a fatty substance produced by supportive brain cells called glial cells. As will be described later, this sheath helps to speed up the movement of neural impulses along the axon.
How Neurons Send Messages Down Their Axons
Neurons exert their influence on other neurons and muscle cells by firing off all-or-none impulses, called action potentials. In motor neurons and interneurons, action potentials are triggered at the junction between the cell body and the axon, and they travel rapidly down the axon to the axon terminals. In sensory neurons they are triggered at the dendritic end of the axon (at upper left in Figure 5.3) and travel through or past the cell body to the axon terminals.
Action potentials are described as “all or none” because they either occur or don’t occur; that is, they don’t partially occur or occur in different sizes or gradations. Each action potential produced by a given neuron is the same strength as any other action potential produced by that neuron, and each action potential retains its full strength all the way down the axon. Although each action potential is all or none, a neuron can convey varying degrees of intensity in its message by varying its rate of producing action potentials. A given neuron might fire off action potentials at a rate anywhere from zero per second to as high as 1,000 per second. By varying its rate of action potentials, a neuron varies the strength of its effect on other neurons or muscle cells.
The Resting Neuron Has a Constant Electrical Charge Across Its Membrane
To understand how action potentials travel down the axon, you have to know something about the functioning of the cell membrane that encloses each neuron. The membrane is a porous “skin” that permits certain chemicals to flow into and out of the cell, while blocking others. Think of the neuron as a tube, the walls of which are the cell membrane. The tube is filled with a solution of water and dissolved chemicals called intracellular fluid and is bathed on the outside by another solution of water and dissolved chemicals called extracellular fluid.
152
3
How does the resting potential arise from the distribution of ions across the cell membrane?
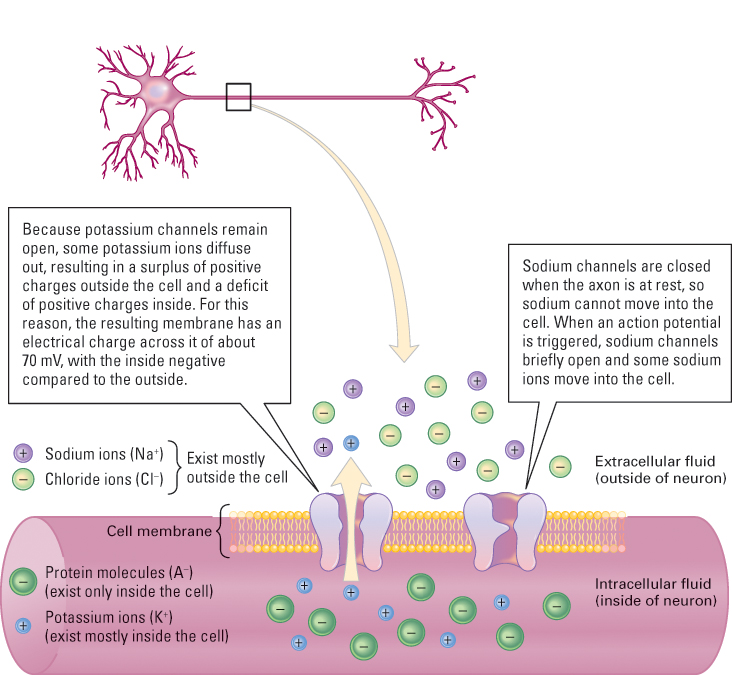
Among the various chemicals dissolved in the intracellular and extracellular fluids are some that have electrical charges. These include soluble protein molecules (A−), which have negative charges and exist only in the intracellular fluid; potassium ions (K+), which are more concentrated in the intracellular than the extra cellular fluid; and sodium ions (Na+) and chloride ions (Cl−), which are more concentrated in the extracellular than the intracellular fluid. For the reason described in Figure 5.5, more negatively charged particles exist inside the cell than outside. This imbalance results in an electrical charge across the membrane, with the inside typically about –70 millivolts (a millivolt [mV] is one-thousandth of a volt) relative to the outside. This charge across the membrane of an inactive neuron is called its resting potential. Just as the charge between the negative and positive poles of a battery is the source of electrical energy in a flashlight, so the resting potential is the source of electrical energy that makes an action potential possible.
The Action Potential Derives from a Brief Change in Membrane Permeability
4
How do the two phases of the action potential (depolarization and repolarization) result from the successive opening and closing of two kinds of channels in the cell membrane?
The action potential is a wave of change in the electrical charge across the axon membrane, and it moves rapidly from one end of the axon to the other. Figure 5.6 depicts an electrical recording of the action potential, over time, at a given location on the axon.
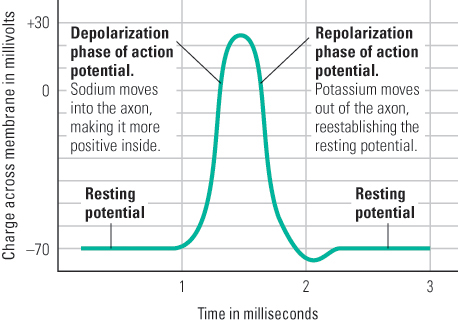
The action potential is initiated by a change in the structure of the cell membrane at one end of the axon: Thousands of tiny channels that permit sodium ions to pass through open up (Figure 5.5). As a result, enough sodium moves inward to cause the electrical charge across the membrane to reverse itself and become momentarily positive inside relative to outside. This sudden shift constitutes the depolarization phase of the action potential (the rising part of the wave shown in Figure 5.6).
153
As soon as depolarization occurs, the channels that permitted sodium to pass through close, but channels that permit potassium to pass through remain open. Because potassium ions are more concentrated inside the cell than outside, and because they are repelled by the temporarily positive environment inside the cell, they are pushed outward. In this process, enough positively charged potassium ions move out of the cell to reestablish the original resting potential. This constitutes the repolarization phase of the action potential. As shown in Figure 5.6, the entire action potential, from depolarization to repolarization, takes about one millisecond (one-thousandth of a second) to occur at any given point on the axon.
With each action potential, a small amount of sodium enters the cell and a small amount of potassium leaves it. To maintain the original balance of these ions across the membrane, each portion of the membrane contains a chemical mechanism, referred to as the sodium-potassium pump, that continuously moves sodium out of the cell and potassium into it.
The Action Potential Regenerates Itself from Point to Point Along the Axon
Action potentials are triggered at one end of an axon by influences that tend to reduce the electrical charge across the cell membrane. In sensory neurons these influences derive from sensory stimuli acting on the dendrites; in motor neurons and interneurons they derive from effects of other neurons that act eventually on the axon at its junction with the cell body.
The axon’s membrane is constructed in such a way that depolarization (reduction in charge across the membrane) to some critical value causes the sodium channels to open, thereby triggering an action potential. This critical value (for example, –65 millivolts inside, compared with a resting potential of –70 millivolts inside) is referred to as the cell’s threshold. Once an action potential occurs at one location on the axon, it depolarizes the area of the axon just ahead of where it is occurring, thus triggering the sodium channels to open there. In this way the action potential keeps renewing itself and moves continuously along the axon. When an axon branches, the action potential follows each branch and thus reaches each of the possibly thousands of axon terminals.
5
How is an axon’s conduction speed related to its diameter and to the presence or absence of a myelin sheath?
The speed at which an action potential moves down an axon is affected by the axon’s diameter. Large-diameter axons present less resistance to the spread of electric currents and therefore conduct action potentials faster than thin ones. Another feature that speeds up the rate of conduction in many axons is the presence of a myelin sheath (refer back to Figure 5.4). Like the plastic cover of an electric wire, myelin protects and insulates axons, speeding the rate at which nervous impulses can be sent and reducing interference from other neurons. Each action potential skips down the axon, from one node to the next, faster than it could move as a continuous wave.
The thickest and most thoroughly myelinated axons in the nervous system can conduct action potentials at a velocity of about 100 meters per second. Thus, it takes about one-hundredth of a second for an action potential to run along that kind of axon from the central nervous system to a muscle about 1 meter away (a finger muscle, for example). Very thin axons without myelin sheaths, in contrast, may conduct at rates as slow as 1 or 2 meters per second. When you poke your finger with a pin, you feel the pressure of the pin before you feel the pain. That is partly because the sensory neurons for pressure are large and myelinated, while those for pain are thin and mostly unmyelinated.
154
The process of developing myelin, called myelination, begins before a child is born but is not complete until some time in adulthood, during the third decade of life or beyond. Neurons in the sensory system are the first to be myelinated, with most sensory structures being completely myelinated before a child’s first birthday. This is followed by myelination of neurons in the motor area, which is nearly complete within the second year of life. The last areas to become fully myelinated are the associative areas in the frontal cortex—the “thinking” area of the brain—which are not completely myelinated until early adulthood (Giedd et al., 1999; Sowell et al., 1999).
How Neurons Are Influenced by Other Neurons: Synaptic Transmission
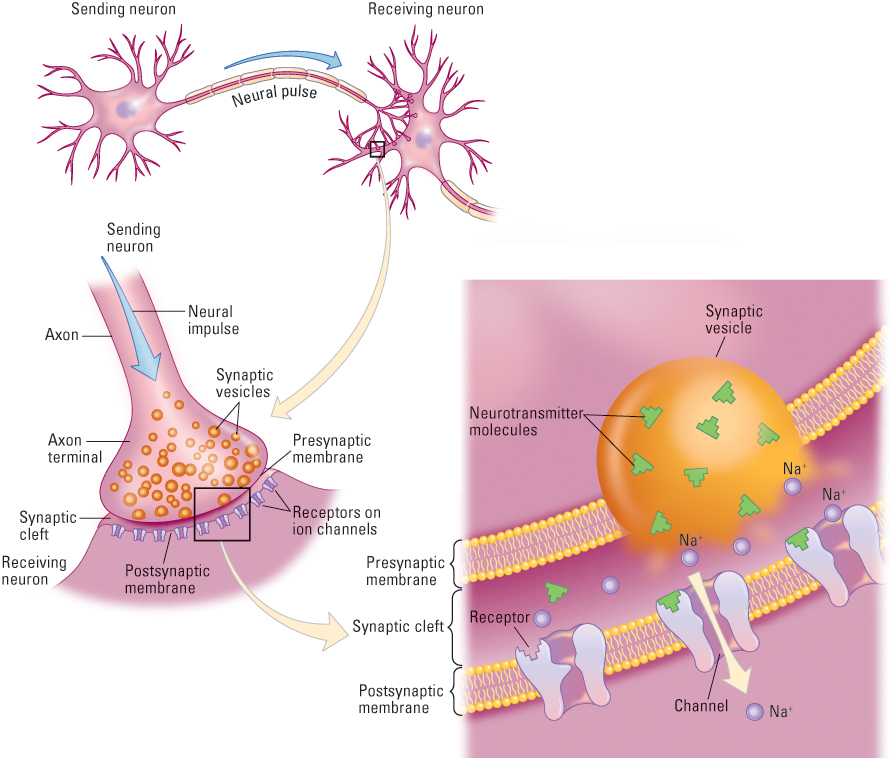
Neurons generate action potentials at rates that are influenced by all the information that is sent to them from other neurons. The cell body and dendrites of a typical motor neuron or interneuron are blanketed by tens of thousands of axon terminals, which come from the branching axons of hundreds or thousands of different neurons (see Figure 5.7). The junction between each axon terminal and the cell body or dendrite of the receiving neuron is referred to as a synapse. When an action potential reaches an axon terminal, it causes the terminal to release packets of a chemical substance, called a neurotransmitter, or transmitter. These chemical substances—which include dopamine, acetylcholine, serotonin, and GABA (gamma-aminobutyric acid), among others—move across the space between the cells and alter the receiving neuron in ways that influence its production of action potentials. Individuals who have too much or too little of some of these neurotransmitters may experience physical and psychological disorders. For example, Parkinson’s disease characterized by muscle tremors and severe difficulty in initiating and coordinating movements—is caused by the degeneration of dopamine-producing neurons whose axons originate and terminate in specific brain areas. Dopamine is also involved in the brain’s reward system and may play an important role in schizophrenia. Other neurotransmitters have similarly been shown to be linked with certain psychological disorders, including serotonin with depression and GABA with anxiety. We will discuss the role of neurotransmitters in psychological disorders in later chapters.
The most fully studied and best-understood synapses are those that exist between axon terminals and muscle cells or between axon terminals and the dendrites of other neurons (see Figure 5.8). A very narrow gap, the synaptic cleft, separates the axon terminal from the membrane of the cell that it influences. The membrane of the axon terminal that abuts the cleft is the presynaptic membrane, and that of the cell on the other side of the cleft is the postsynaptic membrane. Within the axon terminal are hundreds of tiny globe-like vesicles, each of which contains several thousand molecules of a chemical neurotransmitter.
6
How do neurotransmitters at excitatory and inhibitory synapses affect the rate at which action potentials are produced in the postsynaptic neuron?
When an action potential reaches an axon terminal, it causes some of the vesicles to spill their neurotransmitter molecules into the cleft. The molecules then diffuse through the fluid in the cleft, and some become attached to special receptors on the postsynaptic membrane. Each neurotransmitter molecule can be thought of as a key, and each receptor can be thought of as a lock. A molecular key entering a receptor lock opens a gate in the channel, allowing ions to pass through. If the postsynaptic cell is a muscle cell, this flow of ions triggers a biochemical process that causes the cell to contract. If the postsynaptic cell is a neuron, the result is a change in the polarization of that neuron, but the direction of change depends on whether the synapse is excitatory or inhibitory (Byrne, 2003).
At an excitatory synapse (as shown in Figure 5.8), the transmitter opens sodium (Na+) channels in the postsynaptic membrane. The movement of the positively charged sodium ions into the cell causes a slight depolarization of the receiving neuron (the neuron becomes less negative inside), which tends to increase the rate of action potentials triggered in that neuron. At an inhibitory synapse, the transmitter opens either chloride (Cl−) channels or potassium (K+) channels. The movement of negatively charged chloride ions into the cell or of positively charged potassium ions out of the cell causes a slight hyperpolarization of the receiving neuron (the neuron becomes even more negative inside than it was before), which tends to decrease the rate of action potentials triggered in that neuron.
155
Postsynaptic Neurons Integrate Their Excitatory and Inhibitory Inputs
At any given moment, a single neuron may receive input at dozens, hundreds, or even thousands of its fast synapses. Some of these synapses are excitatory and some inhibitory (see Figure 5.9). At each excitatory synapse the transmitter causes a slight depolarization, and at each inhibitory synapse the transmitter causes a slight hyperpolarization. These effects spread passively through the dendrites and cell body, combining to have an integrated effect on the electrical charge across the membrane of the axon at its junction with the cell body.
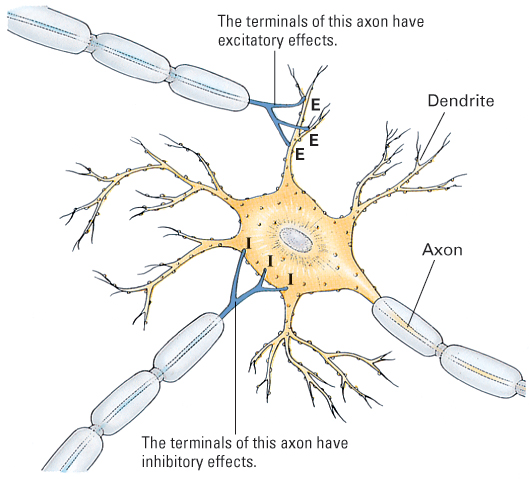
Recall that whenever the axon membrane is depolarized below the critical value, action potentials are triggered. The greater the degree of depolarization below that value, the greater the number of action potentials per second. Thus, the rate of action potentials in the postsynaptic neuron’s axon depends on the net effect of the depolarizing and hyperpolarizing influences from excitatory and inhibitory synapses.
The Development of Neurons
As infants and children get older, their heads and brains get bigger, and they are increasingly able to use their brains to solve problems. It is tempting to think that the number of neurons increases with age, accounting for the increase in brain size and cognition. This is not the case, however. In fact, newborn infants have more neurons in their brains than adults do.
156
Neurogenesis
7
When are most neurons “born” and when do they begin to form synapses?
The process of creating new neurons is referred to as neurogenesis (literally, the birth of neurons), and it occurs during the first 20 weeks after conception, peaking in the third and fourth months of gestation (Lenroot & Giedd, 2007). During its peak, the fetal brain generates several hundred thousand neurons each minute (Nelson et al., 2006). Not long ago, it was believed that all the neurons a person would ever have were generated during the prenatal period. We now know that neurogenesis continues after birth well into adulthood, particularly in the hippocampus, an area involved in memory (Eriksson et al., 1998). We will have more to say about the hippocampus later in this chapter.
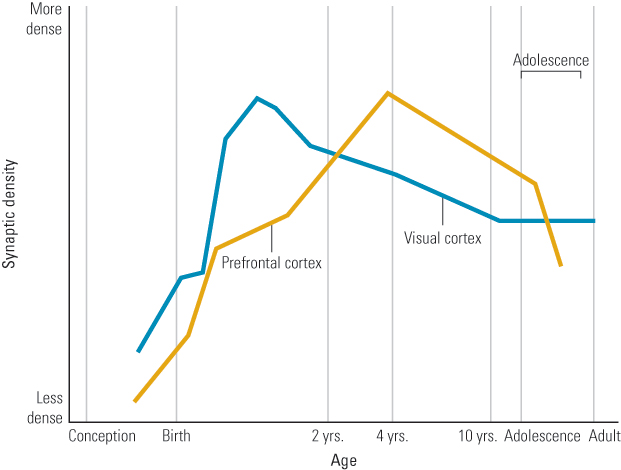
Once neurons are “born,” they migrate to their permanent position in the brain (Bronner Hatten, 2012). Beginning about 20 weeks after conception, they enter the last stage of their development, termed differentiation. During this time, neurons grow in size and increase their numbers of dendrites and axon terminals, as well as the number of synapses they form. Differentiation, of course, doesn’t stop at birth. Synapse formation is most rapid in the months immediately following birth, but the peak of synapse formation varies for different parts of the brain. For example, a burst of synapse formation in the visual cortex begins at about 3 or 4 months and peaks between 4 and 12 months. A similar pattern is found in the prefrontal cortex, but the peak number of synapses is not attained until about 24 months of age (Huttenlocher, 1994).
Cell Death and Synaptic Pruning
8
How can the metaphor of sculpting be applied to brain development?
One perhaps counterintuitive aspect of brain development is that, beginning late in the prenatal period and continuing after birth, the primary changes are in losses of neurons and synapses (Oppenheim et al., 2012; Tapia & Lichtman, 2012). Although brains do get larger with age, this increase is due primarily to the increasing size of individual neurons and myelination of axons, not to the generation of new neurons (Lenroot & Giedd, 2007). In fact, both the number of neurons and the number of synapses actually decrease over early development. At its peak during prenatal development, up to 250,000 synapses are being formed per minute. Yet, 40 to 50 percent of these synapses will be lost, or pruned.
157
Furthermore, it’s not just synapses that are lost; neurons themselves also die in a process, known as selective cell death, or apoptosis [a-pǝp-tō´-sos], which begins before birth and continues well into the teen years (Giedd et al., 1999; Huttenlocher, 1979). Cell death and synaptic pruning occur at different rates for different parts of the brain. For instance, children attain the adult density of visual cortex synapses at 2 to 4 years of age; in contrast, in the prefrontal areas, children continue to have more neurons and synapses than adults into their teen years (Huttenlocher & Dabholkar, 1997; Spear, 2007; see Figure 5.10). Thus, overall, adolescents have fewer but stronger and more effective neuronal connections than they had as children.
Rather than thinking of brain development as simple increases in size and complexity, a better metaphor may be that of sculpting. The brain first overproduces neurons and synapses, but then, just as a sculptor chisels away at extra stone to produce his or her work of art, so too do experience, hormones, and genetic signals shape the brain (Kolb, 1989).
Mirror Neurons: The Basis for Social Learning?
9
What role might mirror neurons play in social learning?
In Chapter 4 we introduced the concept of mirror neurons, found in various parts of the cerebral cortex of monkeys and humans, which have been proposed to play a role in social learning. As you may recall, mirror neurons are active both when a subject engages in a behavior, such as grasping a piece of food, and when the subject observes someone else perform a similar action. Mirror neurons were first discovered in monkeys using recordings from single neurons through electrodes placed in the monkeys’ brains (Rizzolatti & Craighero, 2004; Rizzolatti et al., 1996). Such neurons, it was proposed, may serve as a basis for imitation. However, as we saw in Chapter 4, monkeys do not engage in true imitation. Rather, the apparent function of mirror neurons in monkeys is not to guide reproduction of observed behavior, but to enable the monkey to recognize when another individual’s behavior matches its own. For instance, Annika Paukner and her colleagues (2005) had both monkeys and humans manipulate small cubes with either their hands or their mouths. In some instances, the human imitated the exact behaviors of a monkey, whereas other times the person merely engaged in monkey-like actions, but not the same actions that the monkey was currently doing. The researchers reported that, in most cases, the monkeys preferred to look at the human who was copying them, apparently recognizing that someone else is displaying behavior identical or similar to their own. More generally, mirror neurons reflect an individual being “able to recognize when another is doing something that the self can do” (Byrne, 2005, p. R499).

Although one cannot implant electrodes in people’s brains to detect mirror neurons, the use of transcranial magnetic stimulation (TMS) and functional magnetic resonance imaging (fMRI) allow researchers to look for mirror neurons in humans and discern their purpose. Mirror neurons have indeed been found in humans, and their function seems to be somewhat different from that in monkeys. One important difference is that, unlike those of monkeys, humans’ mirror neurons seem to code for movements forming an action and not only for the action itself (Rizzolatti & Craighero, 2004), suggesting that they are important in imitative learning, where the specific behaviors a model performs (the “means”) are as important as the goal the model attains (the “ends”). The importance of mirror neurons in imitative learning is apparent in a study in which people watched an expert guitarist as he played guitar chords (Buccino et al., 2004). Using fMRI recordings, the researchers found that mirror neurons became activated when subjects watched the expert play chords, but became even more active when they tried to copy the movements.
158
There is still considerable debate about the importance of mirror neurons in social learning (Gallese et al., 2011). However, some believe that they represent a brain-based mechanism for identifying with others (Ramachandran & Oberman, 2006), which is the basis of imitative learning (Iacoboni, 2005), speech perception (D’Ausilio et al., 2009), empathy (Gazzola et al., 2006), and even language (Aziz-Zadeh et al., 2006). Mirror neurons may allow you to understand another’s intentions, which, as we’ll see in subsequent chapters, is the foundation for advanced forms of social cognition. One provocative idea is that changes in the mirror-neuron system permitted greater social-learning abilities in our ancient ancestors, setting the stage for the revolutionary changes in thinking and lifestyle that occurred over relatively brief periods of evolutionary time (Ramachandran & Oberman, 2006; Rizzolatti & Craighero, 2004).
SECTION REVIEW
Neurons—individual nerve cells—“fire” electrochemically and communicate at synapses.
Basic Neural Anatomy
- Interneurons exist entirely within the central nervous system (brain and spinal cord) and carry messages from one set of neurons to another.
- Sensory neurons carry information from sensory organs into the central nervous system.
- Motor neurons carry messages out from the central nervous system to operate muscles and glands.
- A typical neuron has a cell body, dendrites (which receive input to the neuron), and an axon (which carries the neuron’s output).
Basic Functioning of the Neuron
- The resting potential is an electrical imbalance that exists across the neural cell membrane when the neuron is not firing; the inside of the cell is negative relative to the outside.
- An action potential involves a very brief reversal of polarization across the cell membrane, followed by a return to the resting state. These changes sweep down the axon at a speed relative to the axon’s diameter.
- Action potentials are all or none; they do not vary in strength in a given neuron. A neuron’s rate of action potentials does vary, however.
Synapses
- Neurotransmitter molecules released from an axon terminal cross the synaptic cleft to affect another neuron, a gland, or a muscle cell.
- Most synapses have brief, immediate effects—either excitatory or inhibitory—on the postsynaptic neuron.
Neural Development
- Neurogenesis begins prenatally, and newborns have more neurons in their brains than adults.
- Synaptic pruning and selective death of neurons begins prenatally and continues after birth.
Mirror Neurons
- Mirror neurons are found in the cortex of monkeys and humans and are active both when a subject engages in a behavior and when it observes someone else perform a similar action.
- Mirror neurons may be the neurological basis for imitation and social learning.
159