Using the abbreviations of chemical functional groups and molecules enables us to create more compact structures. Alternatively, abbreviated groups can be displayed in their full, expanded forms in favor of a more detailed description of the crucial parts of the molecule.
Marvin for JavaScript offers a wide selection of abbreviated groups belonging to several chemical compound families (carbohydrates, amino acids, etc.). These structures are available under the Abbreviated Groups button of the Tools toolbar. Selecting a group in the opening dialog, you can decide whether to use the group in its contracted or expanded form. For the latter option, tick the checkbox in the dialog window. Pressing the "OK" button closes the dialog and puts the selected group on the tip of the cursor, from where you can place it on the canvas with a left-click.
An abbreviated group can be expanded, contracted, or ungrouped after it has been placed on the canvas. These options are available from the Group pop-up menu.
| • | Expanded groups are denoted by special feedback: blue background and brackets appear around the group if the cursor is over it (the green "hover-over" feedback is still visible on the respective atom or bond), and the abbreviated name is also visible. The attachment points of the groups are circled in blue: |
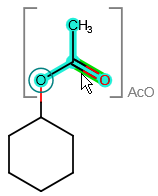
| • | If an expanded group is "ungrouped", the feedback around it will disappear. The same action in case of a contracted group means that the group will be firstly expanded, and its feedback will disappear. |
| • | Contracted groups can be manipulated as atoms; while expanded abbreviated groups can be handled as molecules or fragments: they can be rotated, dragged, merged, mirrored, deleted, and cleaned in 2D. |
| • | Editing an expanded abbreviated group in any sense - i.e., changing/deleting an atom or a bond, modifying the charge or mass number of atoms - leads to the termination of the group status. |
2D cleaning of molecules can be performed using the Clean button of the General toolbar. It calculates new 2D coordinates for the atoms on the canvas in order to get the same molecule in an evenly organized fashion.
Partial cleaning of a structure is possible, too. If you have selected fragments of molecules or certain elements of a multi-molecular structure, 2D cleaning affects only these selected objects.
The 2D cleaning feature is available only if the server connection is active, and the appropriate webservice is available otherwise the Clean button will not appear.
Marvin for JavaScript only highlights valence errors, but does not correct them, so you should make the necessary corrections manually. In case of recognizing a valence error, Marvin for JavaScript highlights the particular atom with red background.
Query structures are generalized structures representing a group of similar molecules which differ only in some structural elements. For example, all of the molecules possess the same skeleton with a halogen atom as substituent, but it is not crucial to know which halogen exactly. See more about query structures in JChem Query Guide. Using query atoms and bonds offered in Marvin for JavaScript different query structures can be designed. Note that atom properties (e.g. atomic charge) can be modified for the query atoms, too.
The "Absolute" chiral flag on a molecule indicates that every chiral center marked with wedge bonds has a known absolute configuration, that is to say, the structure represents a single, well-defined stereoisomer. This chiral flag can be added to a molecule through the pop-up menu and its display can be turned on/off in the View Settings dialog window.
Depending on the file format, a molecule which has wedge bonds without a chiral flag has either of the following meanings:
| • | In MDL file types (MOL, SDF, ...): wedge bonds describe the relative configuration of chiral centers; the structure is a racemic mixture of two enantiomers. |
| • | In Daylight file types (SMILES, SMARTS): wedge bonds describe the absolute configuration of chiral centers; the structure represents a single enantiomer. |
Enhanced stereo specifications
Marvin for JavaScript supports the MDL enhanced stereo representation. Enhanced stereo specifications enable us to refer to several stereoisomers of a molecule by drawing only one structure. Attaching these notations to chiral centers makes it possible to represent stereochemical information: whether we know the absolute configuration of a stereogenic center or we have information only about the relative configuration of two or more chiral atoms. The advantage of enhanced stereo representation is the most palpable when the molecule contains several chiral centers. Illustrating all or even just a few of the molecules stereoisomers can be really demanding in a case like this, but with the help of enhanced stereo specifications only one molecular structure can be enough to represent every diastereomer.
Marvin for JavaScript applies four identifiers, which help us to create groups from the stereocenters. Each group label consists of an identifier, and – in some cases – of a number. Each and every stereocenter belongs only to one stereogenic group. The following identifiers can be attached to stereogenic centers:
| • | Off: We have no information about the configuration of the chiral atom; |
| • | Abs: We know the absolute configuration of the chiral center; |
| • | Or: Attaching the „Or” identifier to a stereogenic center means that we only know the configuration of the atom relative to another chiral center, but we do not have information about the absolute configuration. For a molecule with two chiral centers it either refers to the structure as drawn (e.g., 1R, 2R configuration) or to its enantiomer, which has opposite configuration on its chiral atoms (1S, 2S); |
| • | And: We use the „And” notation if we want to represent a mixture of stereoisomers which can contain specified enantiomers/diastereomers or even every optical isomer. |
You can find a more detailed description of enhanced stereo representation along with a set of examples in the JChem Query Guide.
You can add an "Atom List" query atom to your molecule, which covers a set of atoms. The created structure produces a hit if any of the atoms in the list is found in the target. Similarly, you can use "NOT List" query atoms, too. A NOT List query atom can represent any atom that is not part of the list.
Atom Lists and NOT Lists can be defined using the Periodic Table. Pressing the appropriate button on the dialog then clicking on the atoms one after the other prepares the list. Close the Periodic Table when you are ready: the list of atoms is on the cursor.
Marvin for JavaScript offers the tool to create query structures containing different R-groups. Using the "combo" button on the Atoms toolbar, an R-group with any arbitrary number can be added to a molecule in the same way as atoms are inserted.
A query with an R-group represents substitution variation on the same scaffold. An R-group query can involve several derivatives which differ in one or more substituents.