9.4 Ribosomes
Protein synthesis takes place when tRNA and mRNA molecules associate with ribosomes. The task of the tRNAs and the ribosome is to translate the sequence of nucleotide codons in mRNA into the sequence of amino acids in protein. The term biological machine was used in preceding chapters to characterize multisubunit complexes that perform cellular functions. The replisome, for example, is a biological machine that can replicate DNA with precision and speed. The site of protein synthesis, the ribosome, is much larger and more complex than the machines described thus far. Its complexity is due to the fact that it has to perform several jobs with precision and speed. For this reason, it is better to think of the ribosome as a factory containing many machines that act in concert. Let’s see how this factory is organized to perform its numerous functions.
In all organisms, the ribosome consists of one small and one large subunit, each made up of RNA (called ribosomal RNA or rRNA) and protein. Each subunit is composed of one to three rRNA types and as many as 50 proteins. Ribosomal subunits were originally characterized by their rate of sedimentation when spun in an ultracentrifuge, and so their names are derived from their sedimentation coefficients in Svedberg (S) units, which is an indication of molecular size. In prokaryotes, the small and large subunits are called 30S and 50S, respectively, and they associate to form a 70S particle (Figure 9-10a). The eukaryotic counterparts are called 40S and 60S, and the complete ribosome is called 80S (Figure 9-10b). Although eukaryotic ribosomes are bigger owing to their larger and more numerous components, the components and the steps in protein synthesis are similar overall. The similarities clearly indicate that translation is an ancient process that originated in the common ancestor of eukaryotes and prokaryotes.
Figure 9-10: Protein and RNA molecules compose the two subunits of a ribosome
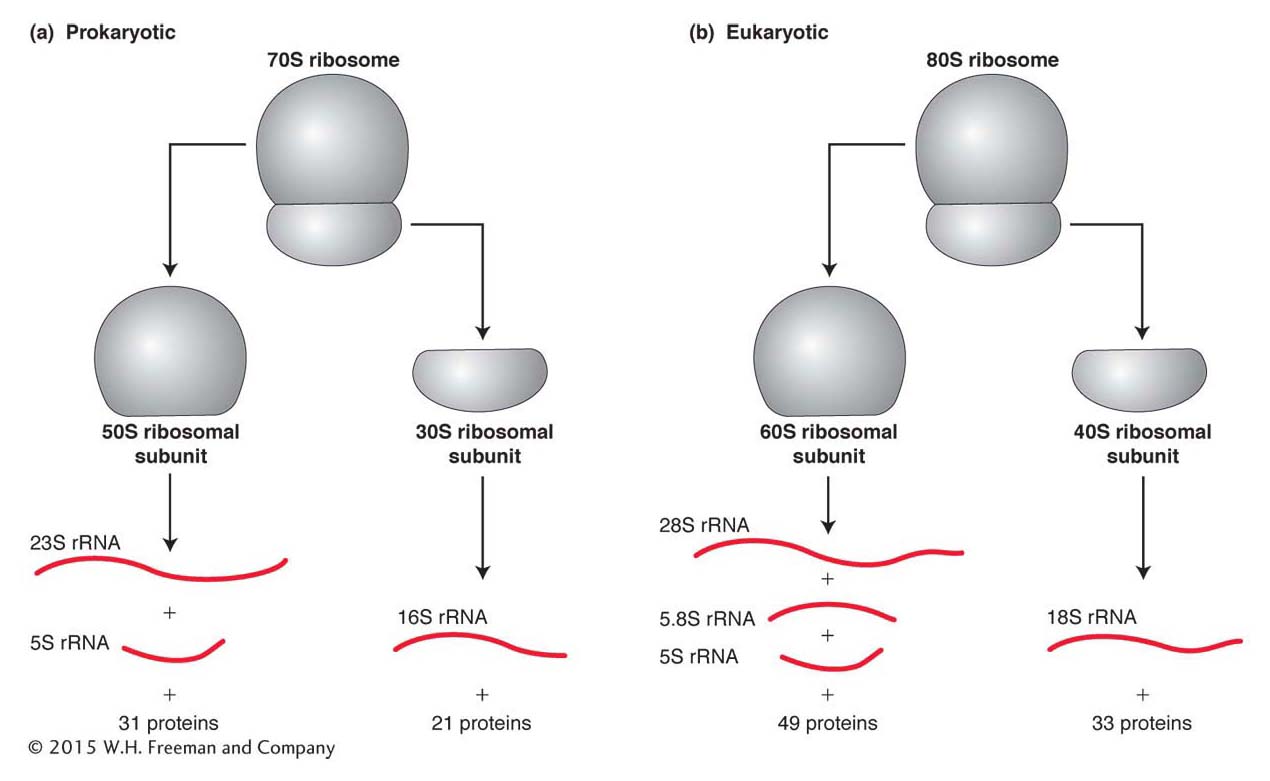
Figure 9-10: A ribosome contains a large and a small subunit. Each subunit contains both rRNA of varying lengths and a set of proteins. There are two principal rRNA molecules in all ribosomes. Prokaryotic ribosomes also contain one 120-base-long rRNA that sediments at 5S, whereas eukaryotic ribosomes have two small rRNAs: a 5S RNA molecule similar to the prokaryotic 5S and a 5.8S molecule 160 bases long.
Page 333
When ribosomes were first studied, the fact that almost two-thirds of their mass is RNA and only one-third is protein was surprising. For decades, rRNAs had been assumed to function as the scaffold or framework necessary for the correct assembly of the ribosomal proteins. That role seemed logical because rRNAs fold up by intramolecular base pairing into stable secondary structures (Figure 9-11). According to this model, the ribosomal proteins were solely responsible for carrying out the important steps in protein synthesis. This view changed with the discovery in the 1980s of catalytic RNAs (see Chapter 8). As you will see, scientists now believe that the rRNAs, assisted by the ribosomal proteins, carry out most of the important steps in protein synthesis.
Figure 9-11: rRNA folds up by intramolecular base pairing
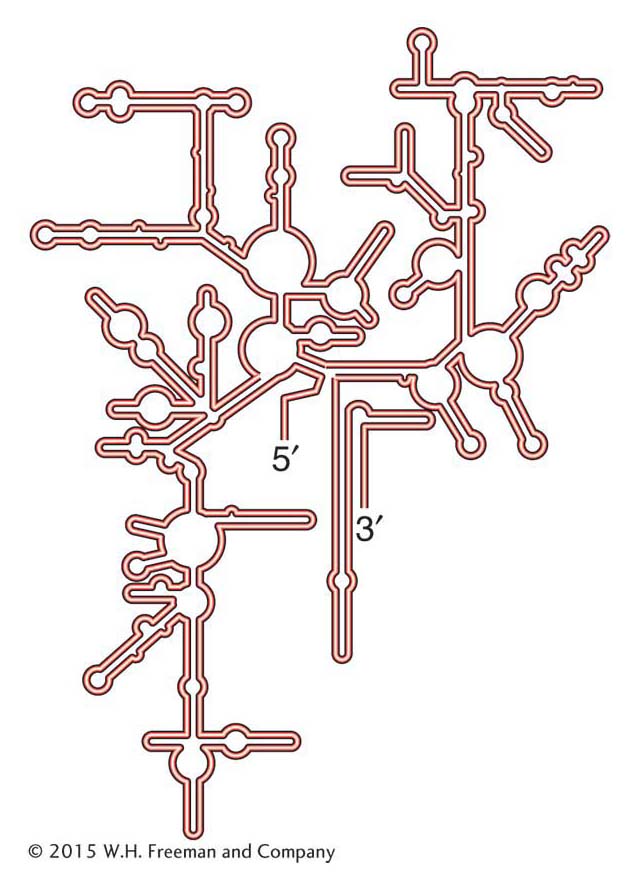
Figure 9-11: The folded structure of the prokaryotic 16S ribosomal RNA of the small ribosomal subunit.
Ribosome features
The ribosome brings together the other important players in protein synthesis— tRNA and mRNA molecules—to translate the nucleotide sequence of an mRNA into the amino acid sequence of a protein. The tRNA and mRNA molecules are positioned in the ribosome so that the codon of the mRNA can interact with the anticodon of the tRNA. The key sites of interaction are illustrated in Figure 9-12. The binding site for mRNA is completely within the small subunit. There are three binding sites for tRNA molecules. Each bound tRNA bridges the 30S and 50S subunits, positioned with its anticodon end in the former and its aminoacyl end (carrying the amino acid) in the latter. The A site (for aminoacyl) binds an incoming aminoacyl-tRNA whose anticodon matches the codon in the A site of the 30S subunit. As we proceed in the 5′ direction on the mRNA, the next codon interacts with the anticodon of the tRNA in the P site (for peptidyl) of the 30S subunit. The tRNA in the P site binds the growing peptide chain, part of which fits into a tunnel-like structure in the 50S subunit. The E site (for exit) contains a deacylated tRNA (it no longer carries an amino acid) that is ready to be released from the ribosome. Whether codon–anticodon interactions also take place between the mRNA and the tRNA in the E site is not clear.
Figure 9-12: Key sites of interaction in the ribosome
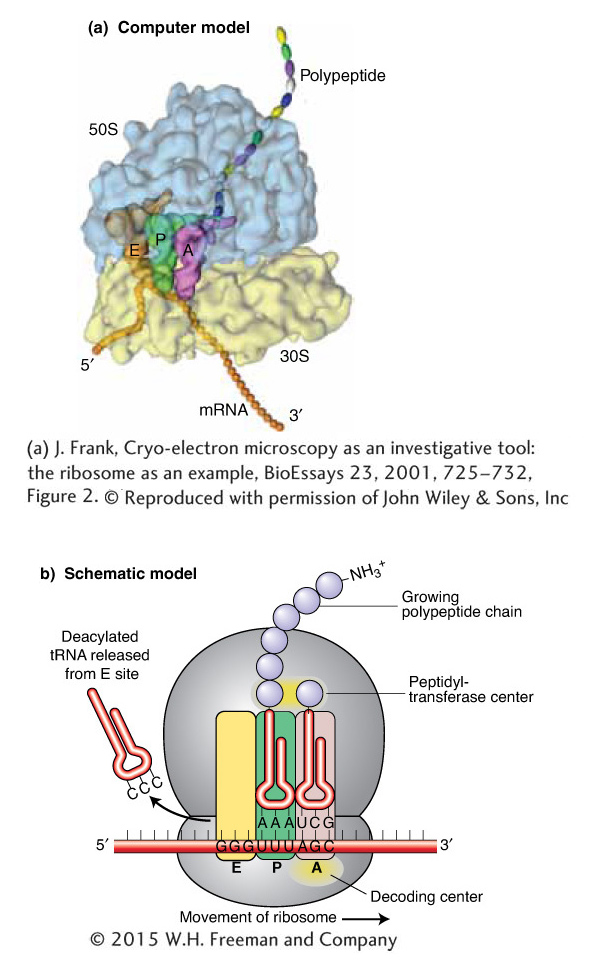
Figure 9-12: Key sites of interaction in a ribosome in the elongation phase of translation. (a) A computer model of the three-dimensional structure of the ribosome including mRNA, tRNAs, and the nascent polypeptide chain as it emerges from the large ribosomal subunit. (b) A schematic model of the ribosome during) translation elongation. See text for details.
[(a) J. Frank, Cryo-electron microscopy as an investigative tool: the ribosome as an example, BioEssays 23, 2001, 725-732, Figure 2. © Reproduced with permission of John Wiley & Sons, Inc.]
Page 334
Two additional regions in the ribosome are critical for protein synthesis. The decoding center in the 30S subunit ensures that only tRNAs carrying anticodons that match the codon (called cognate tRNAs) will be accepted into the A site. The peptidyltransferase center in the 50S subunit is the site where peptide-bond formation is catalyzed. Recently, many laboratories, especially those of Thomas Steitz, Venkatraman Ramakrishnan, and Ada Yonath, have used X-ray crystallography to “solve” the structure of the ribosome at the atomic level. For this accomplishment these three scientists received the Nobel Prize in Chemistry in 2009. The results of their elegant studies clearly show that both the decoding and peptidyltransferase centers are composed entirely of regions of rRNA; that is, the important contacts in these centers are tRNA–rRNA contacts. Peptide-bond formation is even thought to be catalyzed by an active site in the ribosomal RNA and only assisted by ribosomal proteins. In other words, the large ribosomal subunit functions as a ribozyme to catalyze peptide-bond formation.
WHAT GENETICISTS ARE DOING TODAY
Similar structural studies have examined the large ribosomal subunit complexed with several different antibiotics. These studies identified the contact points between the antibiotic and the ribosome and, in doing so, have provided an explanation for why certain antibiotics inactivate only bacterial ribosomes. For example, the macrolides are a family of structurally similar compounds that includes the popular antibiotics erythromycin and Zithromax. These antibiotics inhibit protein synthesis by stalling the ribosome on the mRNA. They do so by binding to a specific region on the 23S rRNA in the large ribosomal subunit and blocking the so-called exit tunnel where the nascent polypeptide emerges from the large subunit (see Figure 9-1). Because of minor sequence differences between the rRNAs of prokaryotes and eukaryotes, macrolides only inhibit bacterial translation. Interestingly, pathogenic bacteria that have evolved resistance to some of these antibiotics appear to have ribosomal mutations that make the exit tunnel larger. Thus, knowledge of how antibiotics bind to ribosomes helps scientists understand how the ribosome works and how to design new antibiotics that can be active against resistant mutants. The use of basic information about cellular machinery to develop new antibiotics and other drugs has been dubbed structure-based drug design.
Page 335
Translation initiation, elongation, and termination
The process of translation can be divided into three phases: initiation, elongation, and termination. Aside from the ribosome, mRNA, and tRNAs, additional proteins are required for the successful completion of each phase. Because certain steps in initiation differ significantly in prokaryotes and eukaryotes, initiation is described separately for the two groups. The elongation and termination phases are described largely as they take place in bacteria, which have been the focus of many recent studies of translation.
Translation initiation The main task of initiation is to place the first aminoacyl-tRNA in the P site of the ribosome and, in this way, establish the correct reading frame of the mRNA. In most prokaryotes and all eukaryotes, the first amino acid in any newly synthesized polypeptide is methionine, specified by the codon AUG. It is inserted not by tRNAMet but by a special tRNA called an initiator, symbolized tRNAMeti. In bacteria, a formyl group is added to the methionine while the amino acid is attached to the initiator, forming N-formylmethionine. (The formyl group on N-formylmethionine is removed later.)
How does the translation machinery know where to begin? In other words, how is the initiation AUG codon selected from among the many AUG codons in an mRNA molecule? Recall that, in both prokaryotes and eukaryotes, mRNA has a 5′ untranslated region consisting of the sequence between the transcriptional start site and the translational start site (see Figure 8-7). As you will see below, the nucleotide sequence of the 5′ UTR adjacent to the AUG initiator is critical for ribosome binding in prokaryotes but not in eukaryotes.
Initiation in prokaryotes Initiation codons are preceded by special sequences called Shine-Dalgarno sequences that pair with the 3′ end of an rRNA, called the 16S rRNA, in the 30S ribosomal subunit (Figure 9-13). This pairing correctly positions the initiator codon in the P site where the initiator tRNA will bind. The mRNA can pair only with a 30S subunit that is dissociated from the rest of the ribosome. Note again that rRNA performs the key function in ensuring that the ribosome is at the right place to start translation.
Figure 9-13: Shine-Dalgarno sequence
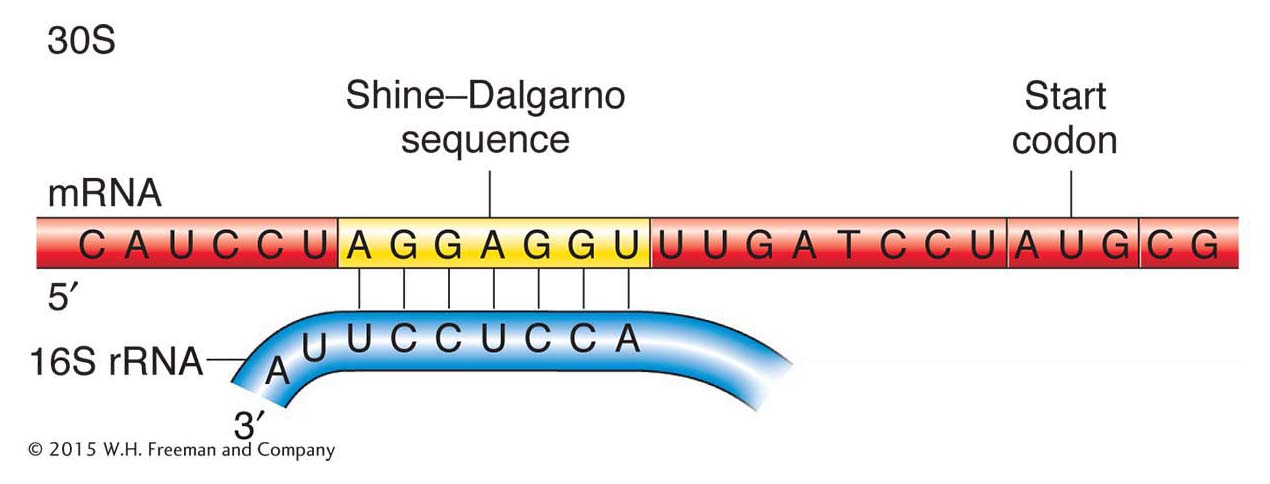
Figure 9-13: In bacteria, base complementarity between the 3′ end of the 16S rRNA of the small ribosomal subunit (30S) and the Shine-Dalgarno sequence of the mRNA positions the ribosome to correctly initiate translation at the downstream AUG codon.
Page 336
Figure 9-14: Translation initiation in prokaryotes
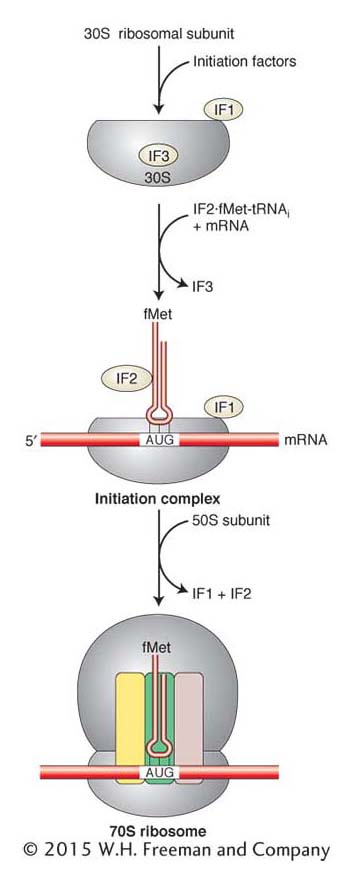
Figure 9-14: Initiation factors assist the assembly of the ribosome at the translation start site and then dissociate before translation.
Three proteins—IF1, IF2, and IF3 (for initiation factor)—are required for correct initiation (Figure 9-14). IF3 is necessary to keep the 30S subunit dissociated from the 50S subunit, and IF1 and IF2 act to ensure that only the initiator tRNA enters the P site. The 30S subunit, mRNA, and initiator tRNA constitute the initiation complex. The complete 70S ribosome is formed by the association of the 50S large subunit with the initiation complex and the release of the initiation factors.
Because a prokaryote lacks a nuclear compartment that separates transcription and translation, the prokaryotic initiation complex is able to form at a Shine–Dalgarno sequence near the 5′ end of an RNA that is still being transcribed. Thus, translation can begin on prokaryotic RNAs even before they are completely transcribed.
Page 337
Initiation in eukaryotes Transcription and translation take place in separate compartments of the eukaryotic cell. As discussed in Chapter 8, eukaryotic mRNAs are transcribed and processed in the nucleus before export to the cytoplasm for translation. On arrival in the cytoplasm, the mRNA is usually covered with proteins, and regions may be double helical due to intramolecular base pairing. These regions of secondary structure must be removed to expose the AUG initiator codon. This removal is accomplished by eukaryotic initiation factors called eIF4A, B, and G. These initiation factors associate with the cap structure (found at the 5′ end of virtually all eukaryotic mRNAs) and with the 40S subunit and initiator tRNA to form an initiation complex. Once in place, the complex moves along the mRNA in the 5′-to-3′ direction and unwinds the base-paired regions (Figure 9-15). At the same time, the exposed sequence is “scanned” for an AUG codon where translation can begin. After the AUG codon is properly aligned with the initiator tRNA, the initiation complex is joined by the 60S subunit to form the 80S ribosome. As in prokaryotes, the eukaryotic initiation factors dissociate from the ribosome before the elongation phase of translation begins.
Figure 9-15: Translation initiation in eukaryotes
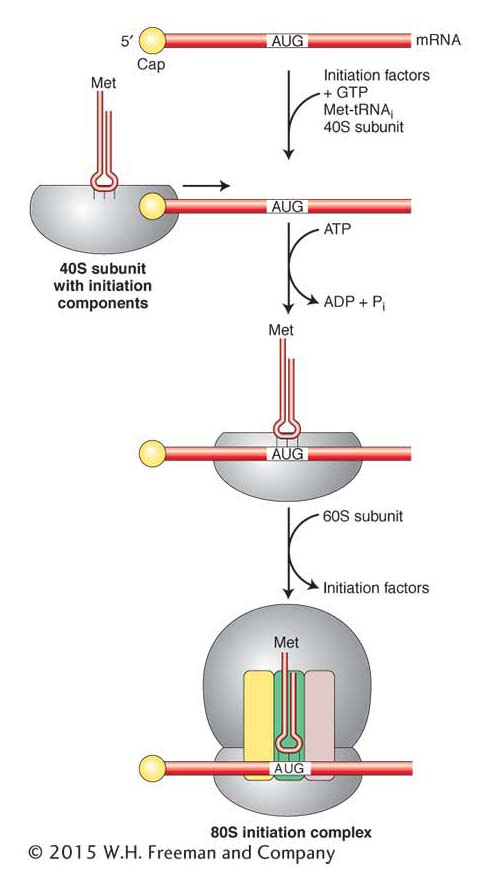
Figure 9-15: The initiation complex forms at the 5′ end of the mRNA and then scans in the 3′ direction in search of a start codon. Recognition of the start codon triggers the assembly of the complete ribosome and the dissociation of initiation factors (not shown). The hydrolysis of ATP provides energy to drive the scanning process.
Elongation It is during the process of elongation that the ribosome most resembles a factory. The mRNA acts as a blueprint specifying the delivery of cognate tRNAs, each carrying as cargo an amino acid. Each amino acid is added to the growing polypeptide chain while the deacylated tRNA is recycled by the addition of another amino acid. Figure 9-16 details the steps in elongation. Two protein factors called elongation factor Tu (EF-Tu) and elongation factor G (EF-G) assist the elongation process.
Figure 9-16: Steps in translation elongation
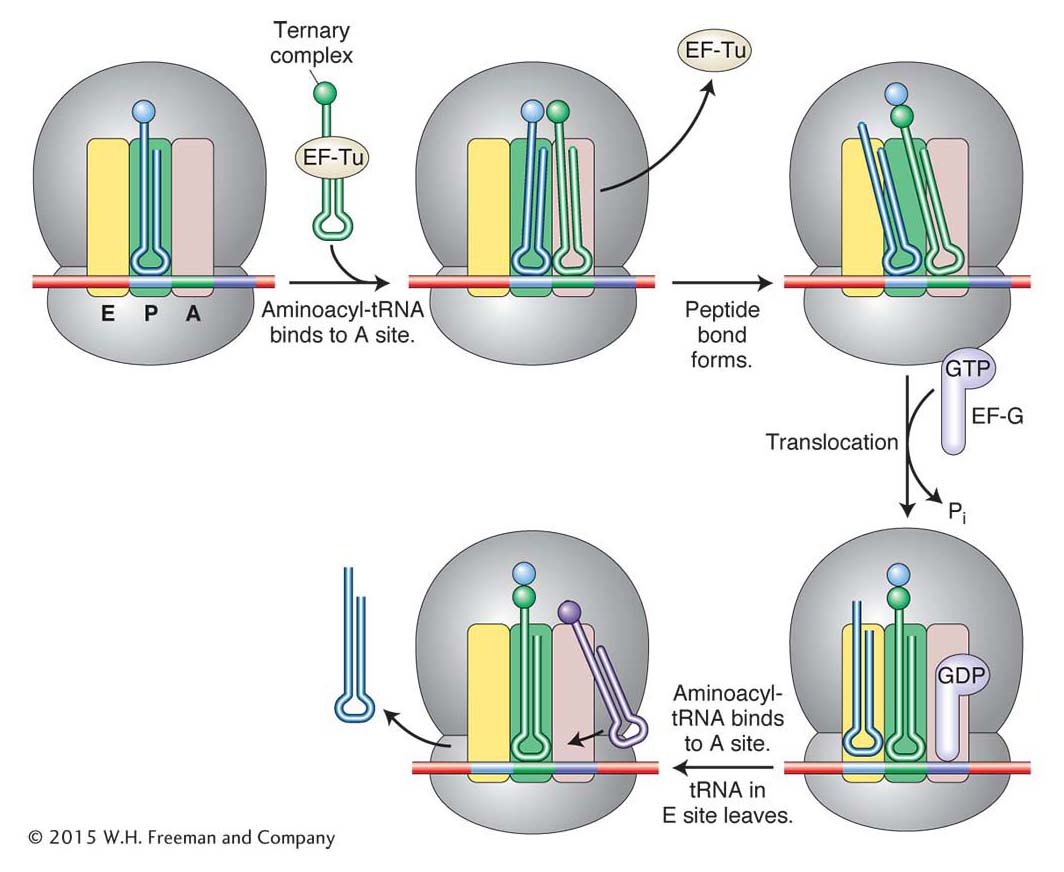
Figure 9-16: A ternary complex consisting of an aminoacyl-tRNA attached to an EF-Tu factor binds to the A site. When its amino acid has joined the growing polypeptide chain, an EF-G factor binds to the A site while nudging the tRNAs and their mRNA codons into the E and P sites. See text for details. ANIMATED ART: The three steps of translation
Page 338
As described earlier in this chapter, an aminoacyl-tRNA is formed by the covalent attachment of an amino acid to the 3′ end of a tRNA that contains the correct anticodon. Before aminoacyl-tRNAs can be used in protein synthesis, they associate with the protein factor EF-Tu to form a ternary complex composed of tRNA, amino acid, and EF-Tu. The elongation cycle commences with an initiator tRNA (and its attached methionine) in the P site and with the A site ready to accept a ternary complex (see Figure 9-16). Which of the 20 different ternary complexes to accept is determined by codon–anticodon recognition in the decoding center of the small subunit (see Figure 9-12b). When the correct match has been made, the ribosome changes shape, the EF-Tu leaves the ternary complex, and the two aminoacyl ends are juxtaposed in the peptidyltransferase center of the large subunit (see Figure 9-12b). There, a peptide bond is formed with the transfer of the methionine in the P site to the amino acid in the A site. At this point, the second protein factor, EF-G, plays its part. The EF-G factor appears to fit into the A site. Its entry into that site shifts the tRNAs in the A and P sites to the P and E sites, respectively, and the mRNA moves through the ribosome so that the next codon is positioned in the A site (see Figure 9-16). When EF-G leaves the ribosome, the A site is open to accept the next ternary complex.
In subsequent cycles, the A site is filled with a new ternary complex as the deacylated tRNA leaves the E site. As elongation progresses, the number of amino acids on the peptidyl-tRNA (at the P site) increases. Eventually, the amino-terminal end of the growing polypeptide emerges from the tunnel in the 50S subunit and protrudes from the ribosome.
Termination The cycle continues until the codon in the A site is one of the three stop codons: UGA, UAA, or UAG. Recall that no tRNAs recognize these codons. Instead, proteins called release factors (RF1, RF2, and RF3 in bacteria) recognize stop codons (Figure 9-17). In bacteria, RF1 recognizes UAA or UAG, whereas RF2 recognizes UAA or UGA; both are assisted by RF3. The interaction between release factors 1 and 2 and the A site differs from that of the ternary complex in two important ways. First, the stop codons are recognized by tripeptides in the RF proteins, not by an anticodon. Second, release factors fit into the A site of the 30S subunit but do not participate in peptide-bond formation. Instead, a water molecule gets into the peptidyltransferase center, and its presence leads to the release of the polypeptide from the tRNA in the P site. The ribosomal subunits separate, and the 30S subunit is now ready to form a new initiation complex.
Figure 9-17: Termination of translation
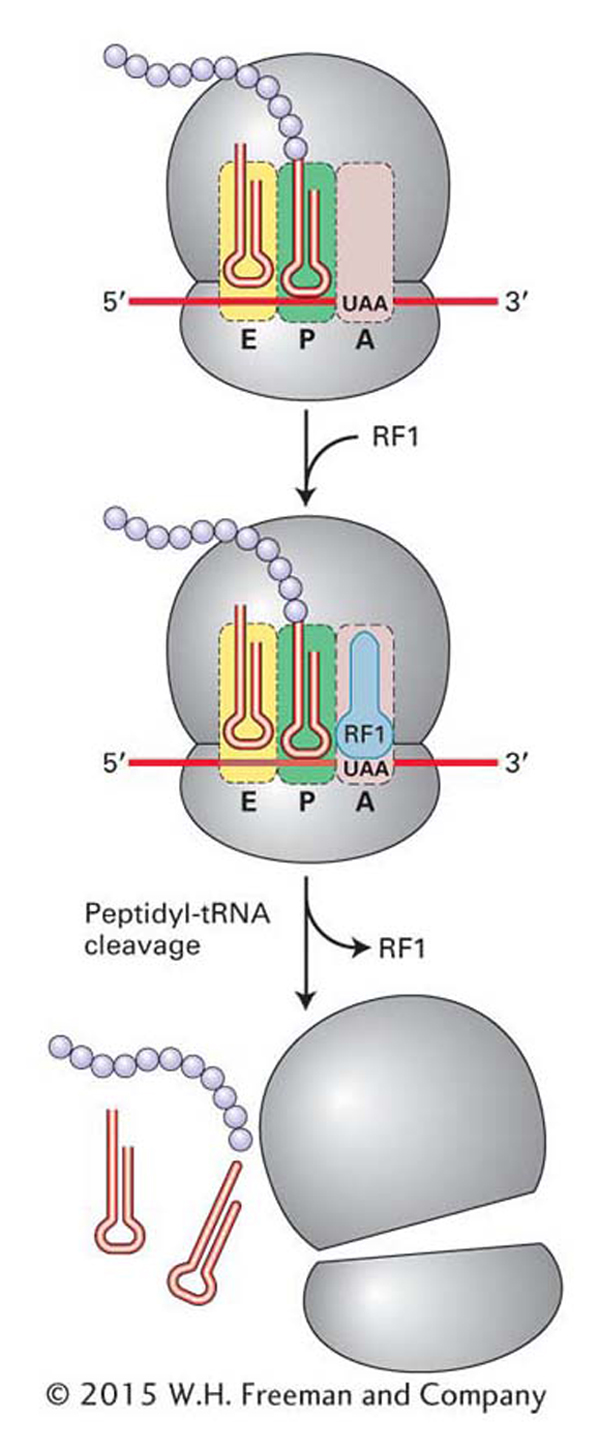
Figure 9-17: Translation is terminated when release factors recognize stop codons in the A site of the ribosome.
KEY CONCEPT
Translation is carried out by ribosomes moving along mRNA in the 5′ → 3′ direction. A set of tRNA molecules bring amino acids to the ribosome, and their anticodons bind to mRNA codons exposed on the ribosome. An incoming amino acid becomes bonded to the amino end of the growing polypeptide chain in the ribosome.
Nonsense suppressor mutations
It is interesting to consider the suppressors of the nonsense mutations defined by Brenner and co-workers. Recall that mutations in phages called amber mutants replaced wild-type codons with stop codons but that suppressor mutations in the host chromosome counteracted the effects of the amber mutations. We can now say more specifically where the suppressor mutations were located and how they worked.
Many of these suppressors are mutations in genes encoding tRNAs and are known as tRNA suppressors. These mutations alter the anticodon loops of specific tRNAs in such a way that a tRNA becomes able to recognize a stop codon in mRNA. In Figure 9-18, the amber mutation replaces a wild-type codon with the chain-terminating stop codon UAG. By itself, the UAG would cause the protein to be prematurely cut off at the corresponding position. The suppressor mutation in this case produces a tRNATyr with an anticodon that recognizes the mutant UAG stop codon. Thus, in the suppressed mutant, tRNATyr competes with the release factor for access to the UAG stop codon. As a result, if tyrosine is inserted, translation continues past that triplet.
Figure 9-18: A suppressor counteracts the effects of a nonsense mutation
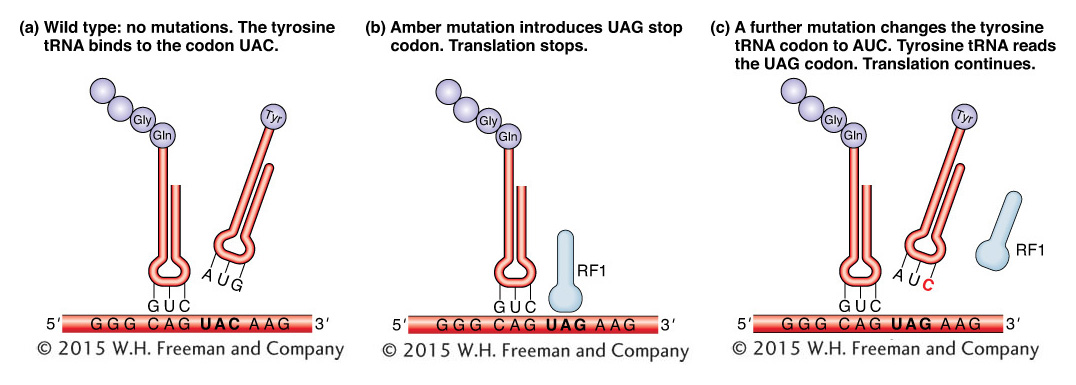
Figure 9-18: A suppressor allows translation to continue when otherwise a mutation would have stopped it. (a) In the wild type, a tRNA reads the codon UAC and translation continues. (b) Termination of translation. Here, the translation apparatus cannot go past a stop codon (UAG in this case), because no tRNA can recognize the UAG triplet. Instead, a release factor binds with the codon and protein synthesis ends, with the subsequent release of the polypeptide fragment. (c) A mutation alters the anticodon of a tyrosine tRNA so that this tRNA can now read the UAG codon. The suppression of the UAG codon by the altered tRNA now permits chain elongation. ANIMATED ART: Nonsense suppression of th e rodns allele, The tRNA nonsense suppressor, The rodns nonsense mutation
Page 339
Could tRNA suppressors (like tRNATyr) also bind to normal termination signals and result in the synthesis of abnormally long proteins? Now that many genomes have been sequenced, it is known that one of the three stop codons, UAA (ochre), is used much more often to terminate protein synthesis. As such, it is not surprising that cells with ochre suppressors are usually sicker than cells with amber or opal suppressor mutations.








