11.1 Gene Regulation
Despite their simplicity of form, bacteria have in common with larger and more complex organisms the need to regulate expression of their genes. One of the main reasons is that they are nutritional opportunists. Consider how bacteria obtain the many important compounds, such as sugars, amino acids, and nucleotides, needed for metabolism. Bacteria swim in a sea of potential nutrients. They can either acquire the compounds that they need from the environment or synthesize them by enzymatic pathways. But synthesizing these compounds also requires expending energy and cellular resources to produce the necessary enzymes for these pathways. Thus, given the choice, bacteria will take compounds from the environment instead. Natural selection favors efficiency and selects against the waste of resources and energy. To be economical, bacteria will synthesize the enzymes necessary to produce compounds only when there is no other option—
Bacteria have evolved regulatory systems that couple the expression of gene products to sensor systems that detect the relevant compound in a bacterium’s local environment. The regulation of enzymes taking part in sugar metabolism provides an example. Sugar molecules can be broken down to provide energy or they can be used as building blocks for a great range of organic compounds. However, there are many different types of sugar that bacteria could use, including lactose, glucose, galactose, and xylose. A different import protein is required to allow each of these sugars to enter the cell. Further, a different set of enzymes is required to process each of the sugars. If a cell were to simultaneously synthesize all the enzymes that it might possibly need, the cell would expend much more energy and materials to produce the enzymes than it could ever derive from breaking down prospective carbon sources. The cell has devised mechanisms to shut down (repress) the transcription of all genes encoding enzymes that are not needed at a given time and to turn on (activate) those genes encoding enzymes that are needed. For example, if only lactose is in the environment, the cell will shut down the transcription of the genes encoding enzymes needed for the import and metabolism of glucose, galactose, xylose, and other sugars. Conversely, E. coli will initiate the transcription of the genes encoding enzymes needed for the import and metabolism of lactose. In sum, cells need mechanisms that fulfill two criteria:
They must be able to recognize environmental conditions in which they should activate or repress the transcription of the relevant genes.
They must be able to toggle on or off, like a switch, the transcription of each specific gene or group of genes.
Let’s preview the current model for prokaryotic transcriptional regulation and then use a well-
The basics of prokaryotic transcriptional regulation: genetic switches
The regulation of transcription depends mainly on two types of protein–
One of these DNA–
The other type of DNA–
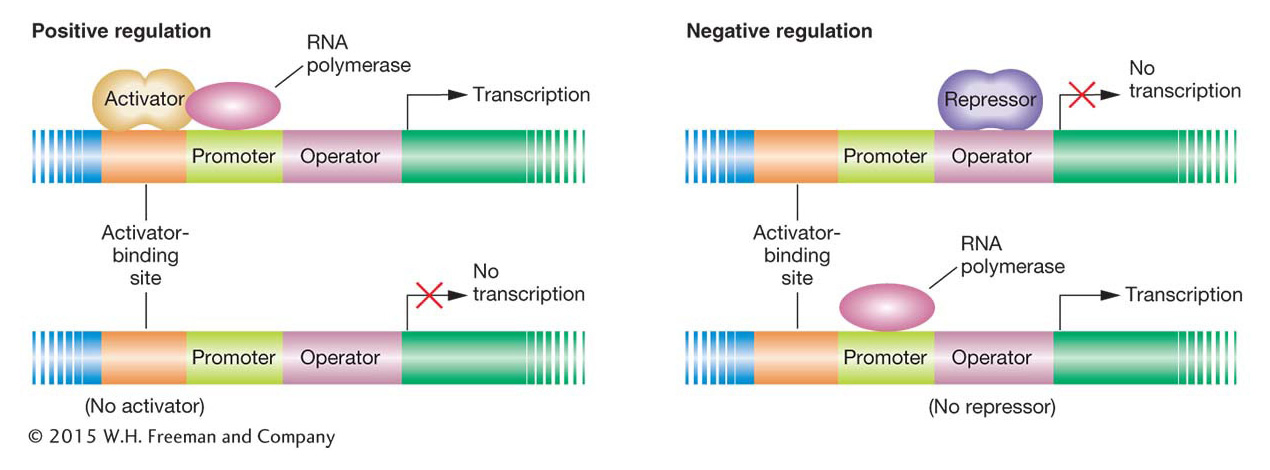
How do activators and repressors regulate transcription? Often, a DNA-
KEY CONCEPT
Genetic switches control gene transcription. The on/off function of the switches depends on the interactions of several proteins with their binding sites on DNA. RNA polymerase interacts with the promoter to begin transcription. Activator or repressor proteins bind to sites in the vicinity of the promoter to control its accessibility to RNA polymerase.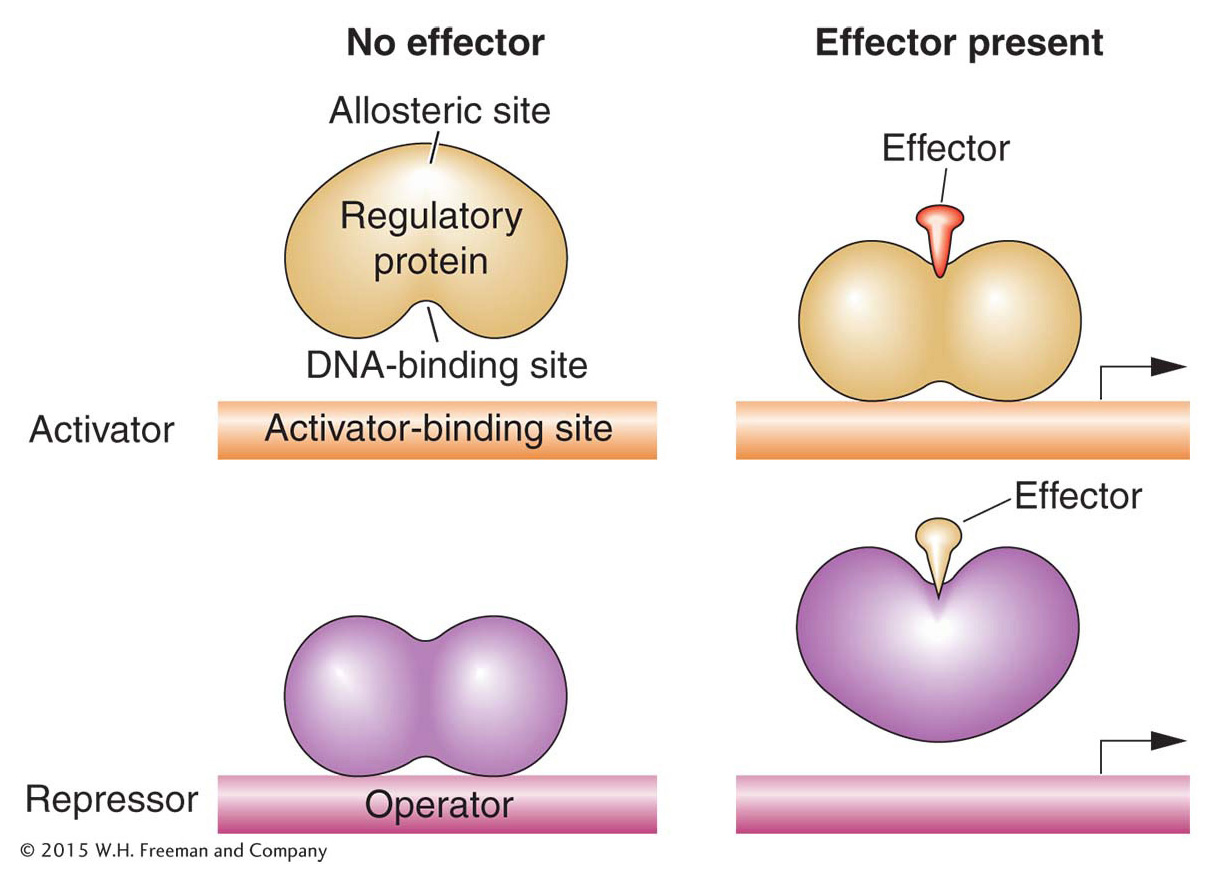
Both activator and repressor proteins must be able to recognize when environmental conditions are appropriate for their actions and act accordingly. Thus, for activator or repressor proteins to do their job, each must be able to exist in two states: one that can bind its DNA targets and another that cannot. The binding state must be appropriate to the set of physiological conditions present in the cell and its environment. For many regulatory proteins, DNA binding is effected through the interaction of two different sites in the three-
In lactose metabolism, it is actually an isomer of the sugar lactose (called allolactose) that is an allosteric effector: the sugar binds to a regulatory protein that inhibits the expression of genes needed for lactose metabolism. In general, an allosteric effector binds to the allosteric site of the regulatory protein in such a way as to change its activity. In this case, allolactose changes the shape and structure of the DNA-
KEY CONCEPT
Allosteric effectors control the ability of activator or repressor proteins to bind to their DNA target sites.A first look at the lac regulatory circuit
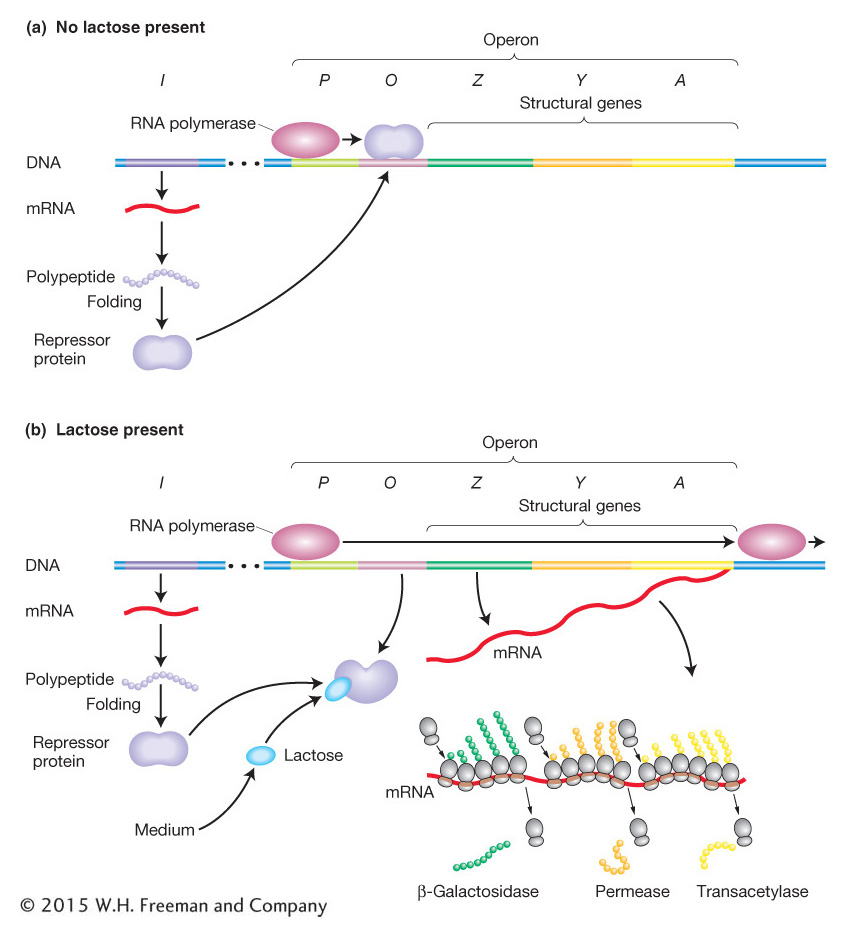
The pioneering work of François Jacob and Jacques Monod in the 1950s showed how lactose metabolism is genetically regulated. Let’s examine the system under two conditions: the presence and the absence of lactose. Figure 11-4 is a simplified view of the components of this system. The cast of characters for lac operon regulation includes protein-
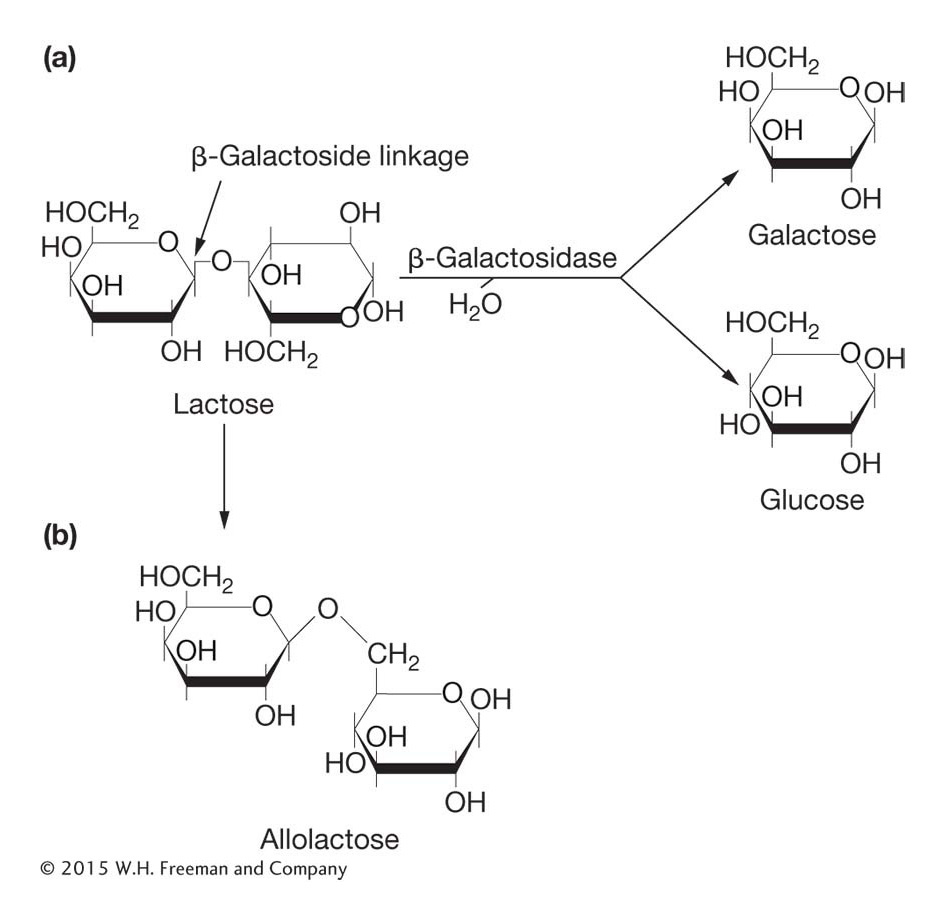
The lac structural genes The metabolism of lactose requires two enzymes: (1) a permease to transport lactose into the cell and (2) β-galactosidase to modify lactose into allolactose and to cleave the lactose molecule to yield glucose and galactose (Figure 11-5). The structures of the β-galactosidase and permease proteins are encoded by two adjacent sequences, Z and Y, respectively. A third contiguous sequence encodes an additional enzyme, termed transacetylase, which is not required for lactose metabolism. We will call Z, Y, and A structural genes—
KEY CONCEPT
If the genes encoding proteins constitute a single transcription unit, the expression of all these genes will be coordinately regulated.Regulatory components of the lac system Key regulatory components of the lactose metabolic system include a gene encoding a transcription regulatory protein and two binding sites on DNA: one site for the regulatory protein and another site for RNA polymerase.
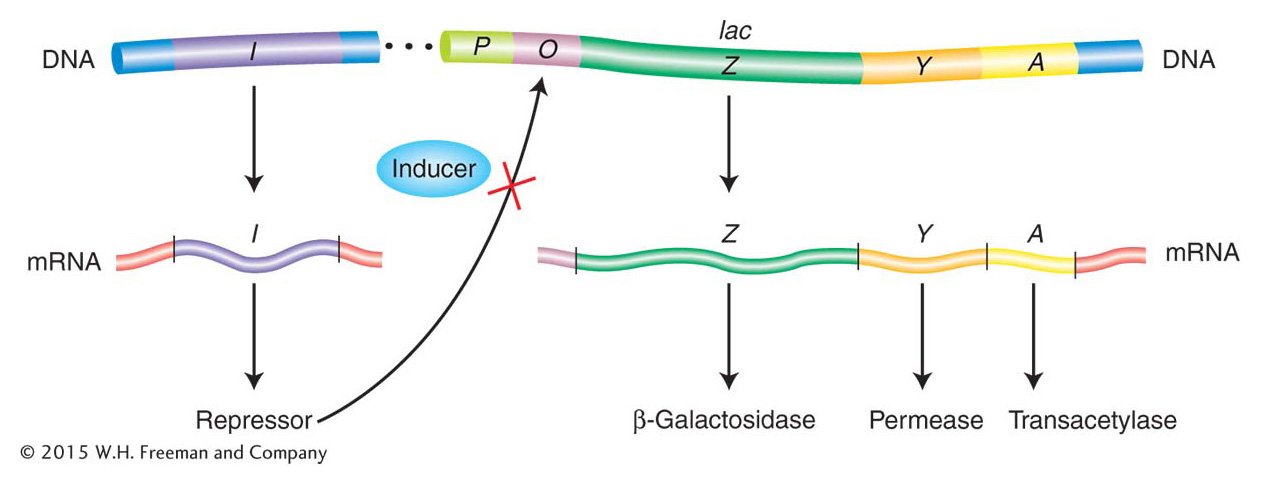
The gene for the Lac repressor. A fourth gene (besides the structural genes Z, Y, and A), the I gene, encodes the Lac repressor protein. It is so named because it can block the expression of the Z, Y, and A genes. The I gene happens to map close to the Z, Y, and A genes, but this proximity is not important to its function because it encodes a diffusible protein.
The lac promoter site. The promoter (P) is the site on the DNA to which RNA polymerase binds to initiate transcription of the lac structural genes (Z, Y, and A).
The lac operator site. The operator (O) is the site on the DNA to which the Lac repressor binds. It is located between the promoter and the Z gene near the point at which transcription of the multigenic mRNA begins.
The induction of the lac system The P, O, Z, Y, and A segments (shown in Figure 11-6) together constitute an operon defined as a segment of DNA that encodes a multigenic mRNA as well as an adjacent common promoter and regulatory region. The lacI gene, encoding the Lac repressor, is not considered part of the lac operon itself, but the interaction between the Lac repressor and the lac operator site is crucial to proper regulation of the lac operon. The Lac repressor has a DNA-
ANIMATED ART: Assaying lactose presence or absence through the Lac repressor
When allolactose or its analogs bind to the repressor protein, the protein undergoes an allosteric transition, a change in shape. This slight alteration in shape in turn alters the DNA-
Let’s summarize how the lac switch works. In the absence of an inducer (allolactose or an analog), the Lac repressor binds to the lac operator site and prevents transcription of the lac operon by blocking the movement of RNA polymerase. In this sense, the Lac repressor acts as a roadblock on the DNA. Consequently, all the structural genes of the lac operon (the Z, Y, and A genes) are repressed, and there are very few molecules of β-galactosidase, permease, or transacetylase in the cell. In contrast, when an inducer is present, it binds to the allosteric site of each Lac repressor subunit, thereby inactivating the site that binds to the operator. The Lac repressor falls off the DNA, allowing the transcription of the structural genes of the lac operon to begin. The enzymes β-galactosidase, permease, and transacetylase now appear in the cell in a coordinated fashion. So, when lactose is present in the environment of a bacterial cell, the cell produces the enzymes needed to metabolize it. But when no lactose is present, resources are not wasted.