15.3 Transposable Elements in Eukaryotes
Although transposable elements were first discovered in maize, the first eukaryotic elements to be characterized at the molecular level were isolated from mutant yeast and Drosophila genes. Eukaryotic transposable elements fall into two classes: class 1 retrotransposons and class 2 DNA transposons. The first class to be isolated, the retrotransposons, are not at all like the prokaryotic IS elements and transposable elements.
Class 1: retrotransposons
The laboratory of Gerry Fink was among the first to use yeast as a model organism to study eukaryotic gene regulation. Through the years, he and his colleagues isolated thousands of mutations in the HIS4 gene, which encodes one of the enzymes in the pathway leading to the synthesis of the amino acid histidine.
They isolated more than 1500 spontaneous HIS4 mutants and found that two of them had an unstable mutant phenotype. The unstable mutants (called pseudorevertants) were more than 1000 times as likely to revert to a phenotype that was similar to wild type as the other HIS4 mutants. Symbolically, we say that these unstable mutants reverted from His− to His+ (wild types have a superscript plus sign, whereas mutants have a superscript minus sign). Like the E. coli gal– mutants, these yeast mutants were found to harbor a large DNA insertion in the HIS4 gene. The insertion turned out to be very similar to one of a group of transposable elements already characterized in yeast, called the Ty elements. There are, in fact, about 35 copies of the inserted element, called Ty1, in the yeast genome.
Cloning of the elements from these mutant alleles led to the surprising discovery that the insertions did not look at all like bacterial IS elements or transposons. Instead, they resembled a well-
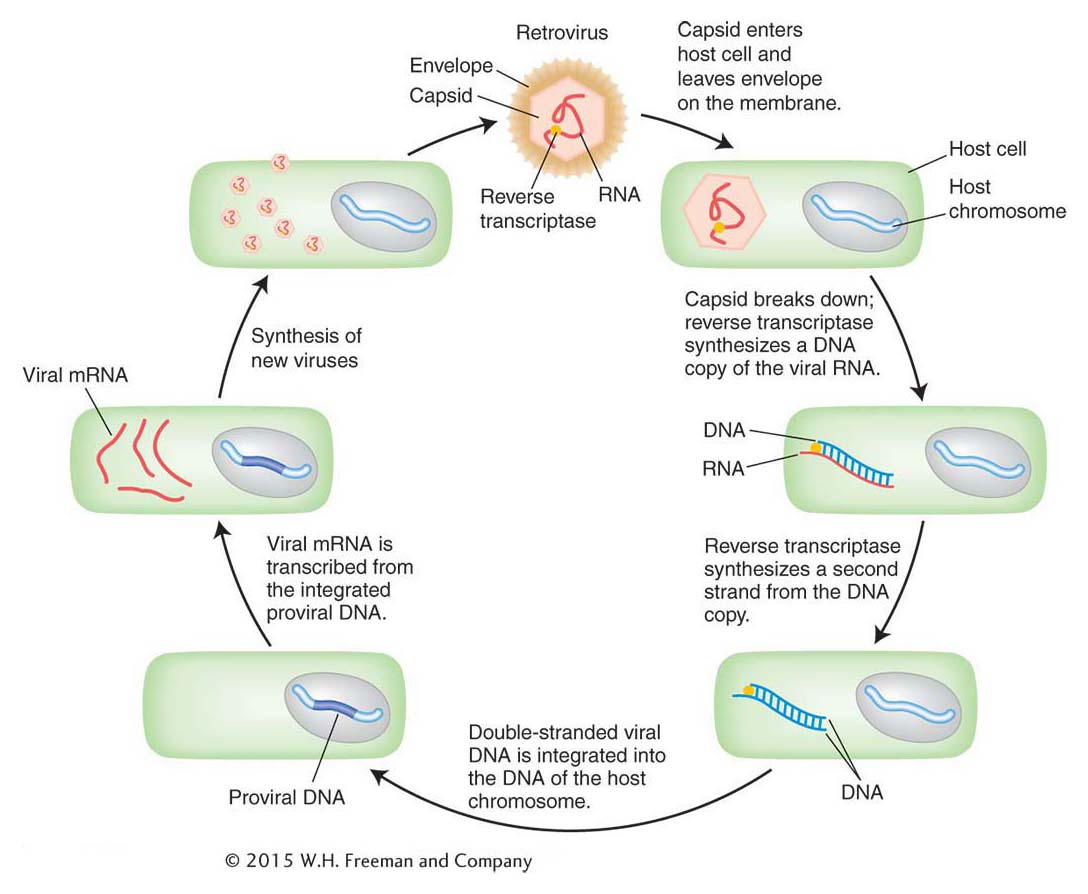
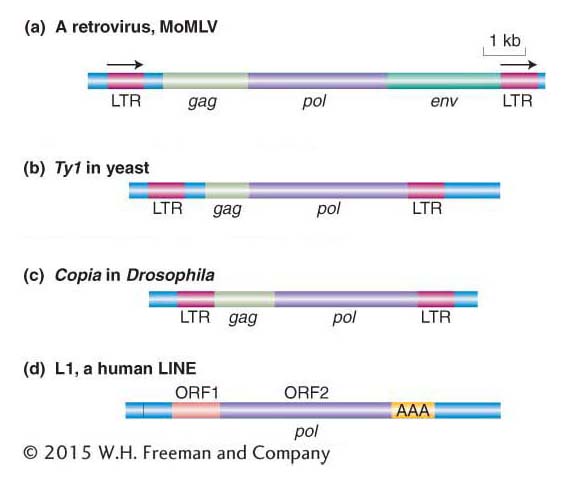
Figure 15-12 shows the similarity in structure and gene content of a retrovirus and the Ty1 element isolated from the HIS4 mutants. Both are flanked by long terminal repeat (LTR) sequences that are several hundred base pairs long. Retroviruses encode at least three proteins that take part in viral replication: the products of the gag, pol, and env genes. The gag-encoded protein has a role in the maturation of the RNA genome, pol encodes the all-
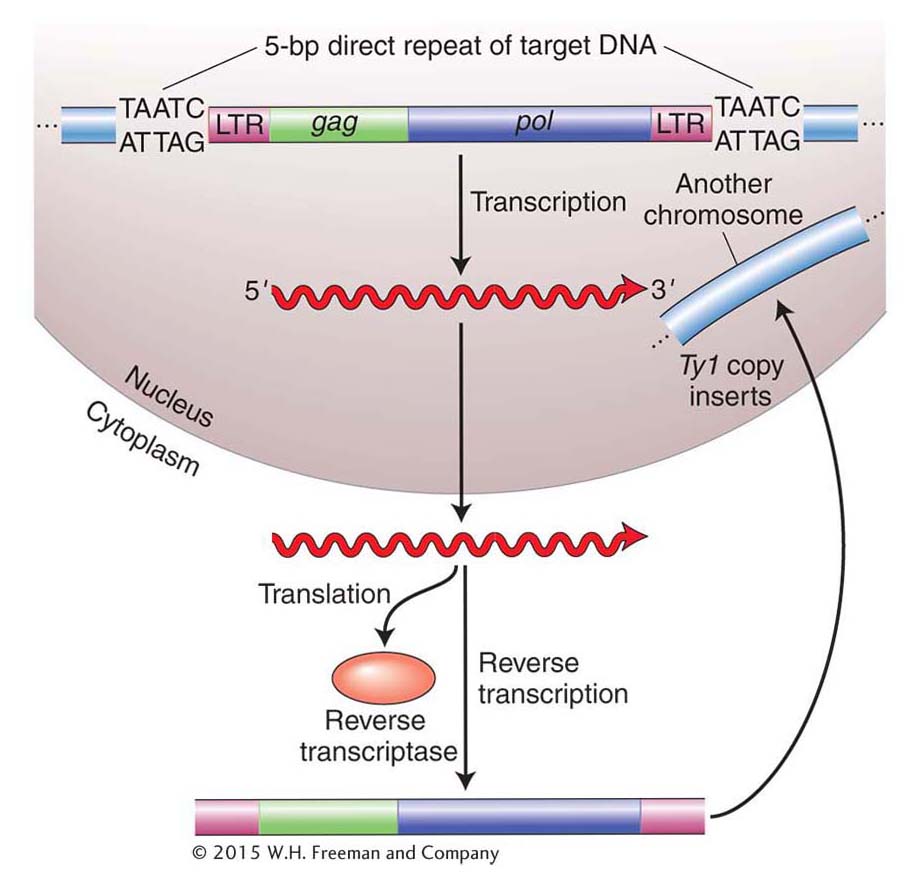
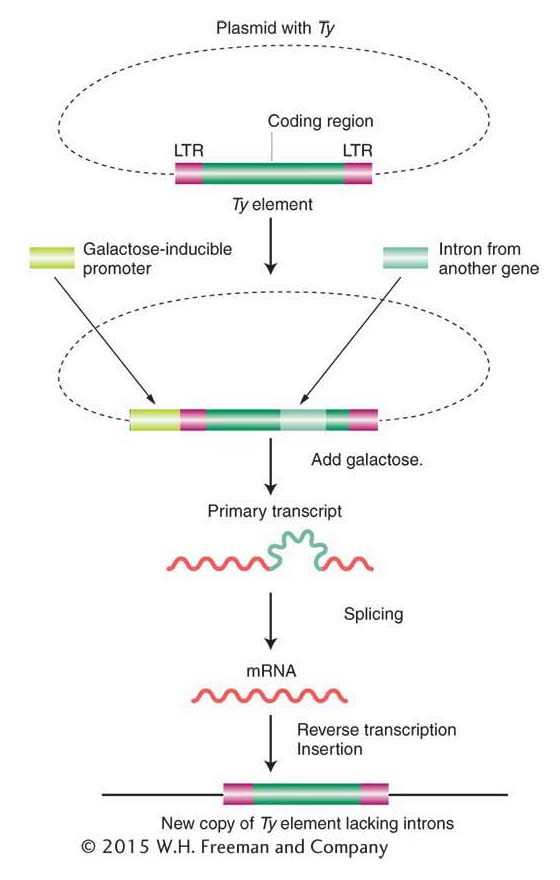
In 1985, David Garfinkel, Jef Boeke, and Gerald Fink showed that, like retroviruses, Ty elements do in fact transpose through an RNA intermediate. Figure 15-14 diagrams their experimental design. They began by altering a yeast Ty1 element, cloned on a plasmid. First, near one end of an element, they inserted a promoter that can be activated by the addition of galactose to the medium. Second, they introduced an intron from another yeast gene into the coding region of the Ty transposon.
The addition of galactose greatly increases the frequency of transposition of the altered Ty element. This increased frequency suggests the participation of RNA because galactose stimulates the transcription of Ty DNA into RNA, beginning at the galactose-
Several spontaneous mutations isolated through the years in Drosophila also were shown to contain retrotransposon insertions. The copia-like elements of Drosophila are structurally similar to Ty1 elements and appear at 10 to 100 positions in the Drosophila genome (see Figure 15-12c). Certain classic Drosophila mutations result from the insertion of copia-like and other elements. For example, the white-
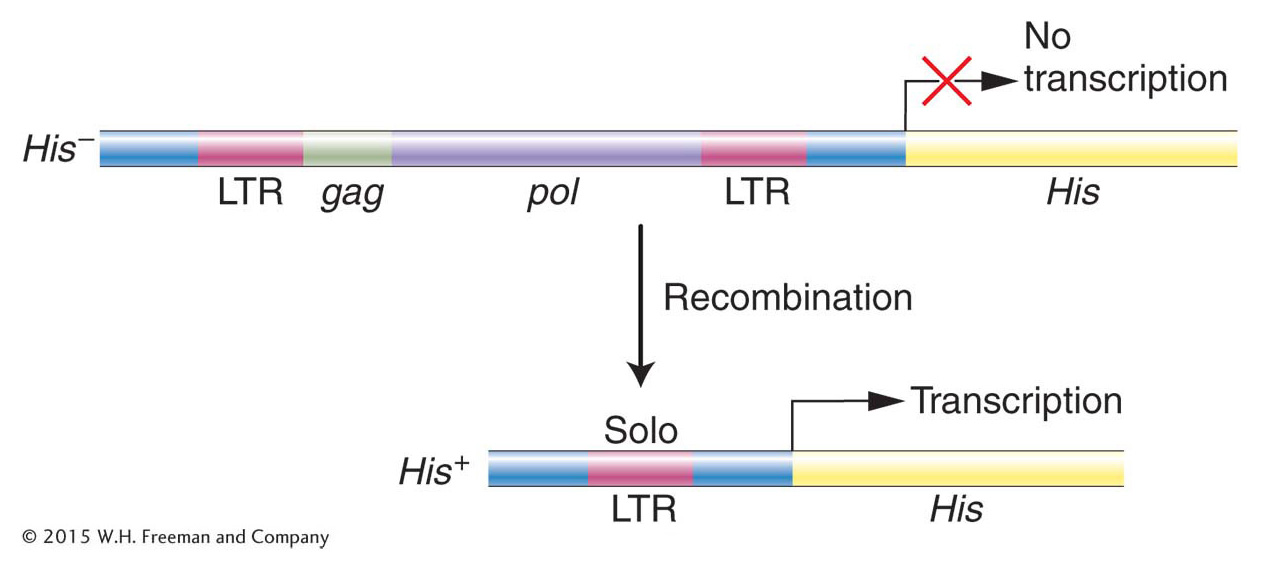
Before we leave retrotransposons (we will return to them later in this chapter), there is one question that needs to be answered. Recall that the first LTR-
KEY CONCEPT
Transposable elements that transpose through RNA intermediates predominate in eukaryotes. Retrotransposons, also known as class 1 elements, encode a reverse transcriptase that produces a double-Class 2: DNA transposons
Some mobile elements found in eukaryotes appear to transpose by mechanisms similar to those in bacteria. As illustrated in Figure 15-8 for IS elements and transposons, the entity that inserts into a new position in the genome is either the element itself or a copy of the element. Elements that transpose in this manner are called class 2 elements, or DNA transposons. The first transposable elements discovered by McClintock in maize are now known to be DNA transposons. However, the first DNA transposons to be molecularly characterized were the P elements in Drosophila.
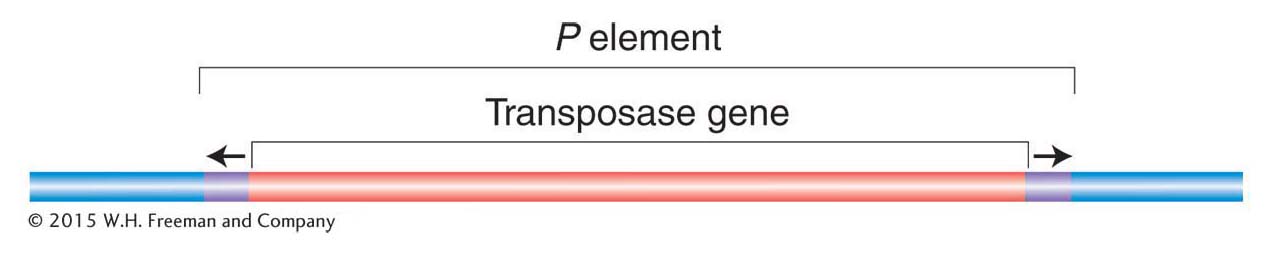
P elements Of all the transposable elements in Drosophila, the most intriguing and useful to geneticists are the P elements. The full-
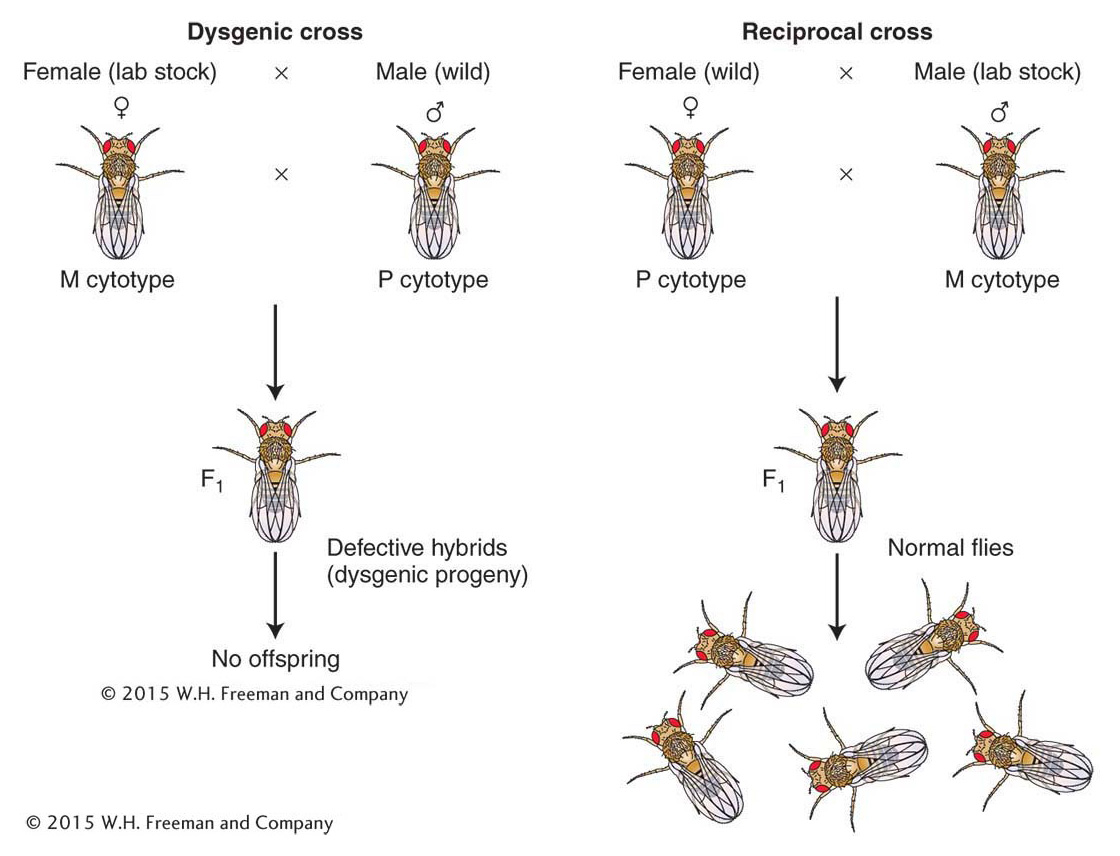
P elements were discovered by Margaret Kidwell, who was studying hybrid dysgenesis—a phenomenon that occurs when females from laboratory strains of D. melanogaster are mated with males derived from natural populations. In such crosses, the laboratory stocks are said to possess an M cytotype (cell type), and the natural stocks are said to possess a P cytotype. In a cross of M (female) × P (male), the progeny show a range of surprising phenotypes that are manifested in the germ line, including sterility, a high mutation rate, and a high frequency of chromosomal aberration and nondisjunction (Figure 15-17). These hybrid progeny are dysgenic, or biologically deficient (hence, the expression hybrid dysgenesis). Interestingly, the reciprocal cross, P (female) × M (male), produces no dysgenic offspring. An important observation is that a large percentage of the dysgenically induced mutations are unstable; that is, they revert to wild type or to other mutant alleles at very high frequencies. This instability is generally restricted to the germ line of an individual fly possessing an M cytotype by a mechanism explained below.
The unstable Drosophila mutants had similarities to the unstable maize mutants characterized by McClintock. Investigators hypothesized that the dysgenic mutations are caused by the insertion of transposable elements into specific genes, thereby rendering them inactive. According to this view, reversion would usually result from the excision of these inserted sequences. This hypothesis has been critically tested by isolating unstable dysgenic mutations at the eye-
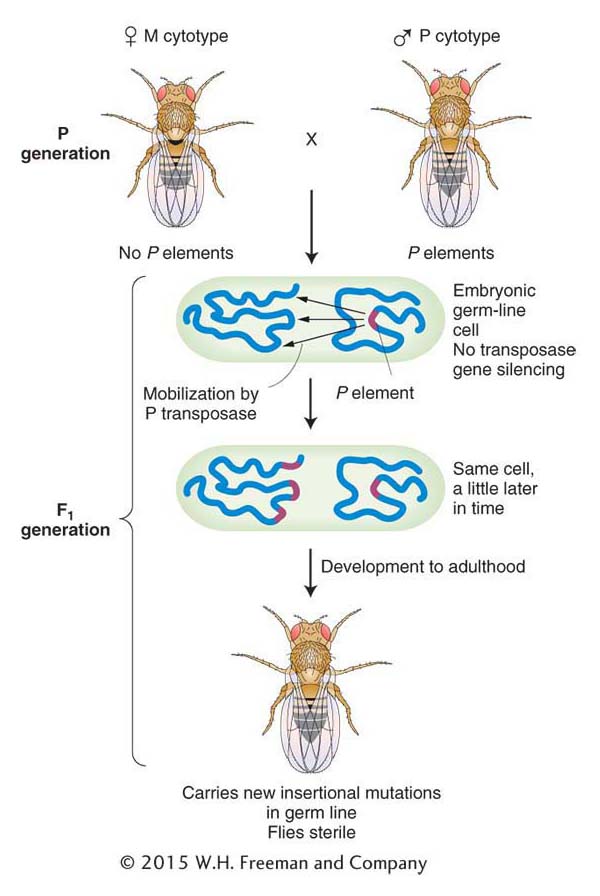
Why do P elements not cause trouble in P strains? The simple answer is that P-element transposition is repressed in P strains. At first it was thought that repression was due to a protein repressor that was in P but not M strains. This model is no longer favored. Instead, geneticists now think that all the transposase genes in P elements are silenced in P strains. The genes are activated in the F1 generation as shown in Figure 15-18. Gene silencing has been discussed previously (see Chapters 8 and 12) and will be revisited at the end of this chapter. For some reason, most laboratory strains have no P elements, and consequently the silencing mechanism is not activated. In hybrids from the cross M (female, no P elements) × P (male, P elements), the P elements in the newly formed zygote are in a silencing-
An intriguing question remains unanswered: Why do laboratory strains lack P elements, whereas strains in the wild have P elements? One hypothesis is that most of the current laboratory strains descended from the original isolates taken from the wild by Morgan and his students almost a century ago. At some point between the capture of those original strains and the present, P elements spread through natural populations but not through laboratory strains. This difference was not noticed until wild strains were again captured and mated with laboratory strains.
Although we don’t know exactly how P elements have spread, it is clear that transposable elements can spread rapidly from a few individual members of a population. In this regard, the spread of P elements resembles the spread of transposons carrying resistance genes to formerly susceptible bacterial populations.
Maize transposable elements revisited Although the causative agent responsible for unstable mutants was first shown genetically to be transposable elements in maize, it was almost 50 years before the maize Ac and Ds elements were isolated and shown to be related to DNA transposons in bacteria and in other eukaryotes. Like the P element of Drosophila, Ac has terminal inverted repeats and encodes a single protein, the transposase. The nonautonomous Ds element does not encode transposase and thus cannot transpose on its own. When Ac is in the genome, its transposase can bind to both ends of Ac or Ds elements and promote their transposition (Figure 15-19).
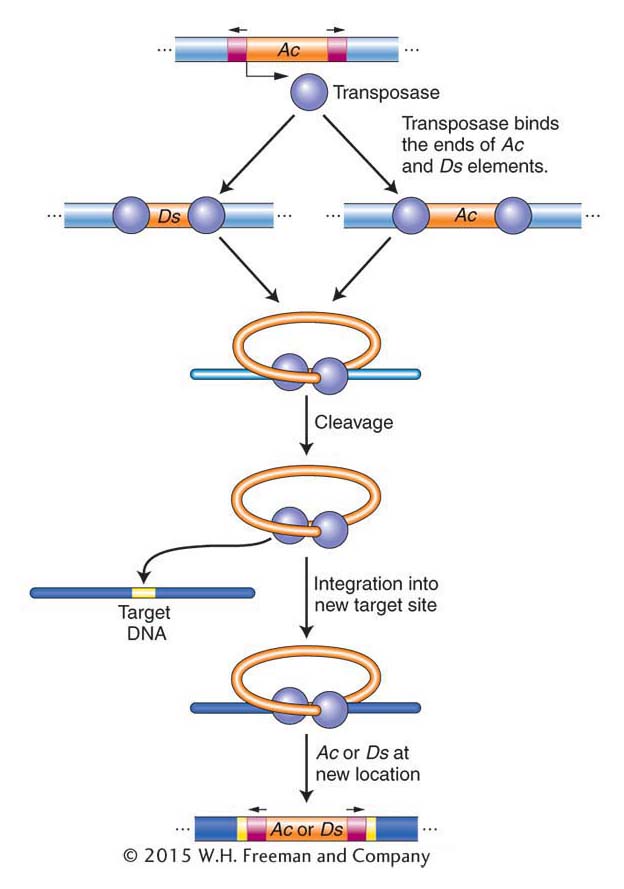
As noted earlier in the chapter, Ac and Ds are members of a single transposon family, and there are other families of transposable elements in maize. Each family contains an autonomous element encoding a transposase that can move elements in the same family but cannot move elements in other families because the transposase can bind only to the ends of family members.
Although some organisms such as yeast have no DNA transposons, elements structurally similar to the P and Ac elements have been isolated from many plant and animal species.
KEY CONCEPT
The first known transposable elements in maize are DNA transposons that structurally resemble DNA transposons in bacteria and other eukaryotes. DNA transposons encode a transposase that cuts the transposon from the chromosome and catalyzes its reinsertion at other chromosomal locations.Utility of DNA transposons for gene discovery
Quite apart from their interest as a genetic phenomenon, DNA transposons have become major tools used by geneticists working with a variety of organisms. Their mobility has been exploited to tag genes for cloning and to insert transgenes. The P element in Drosophila provides one of the best examples of how geneticists exploit the properties of transposable elements in eukaryotes.
Using P elements to tag genes for cloning P elements can be used to create mutations by insertion, to mark the position of genes, and to facilitate the cloning of genes. P elements inserted into genes in vivo disrupt genes at random, creating mutants with different phenotypes. Fruit flies with interesting mutant phenotypes can be selected for cloning of the mutant gene, which is marked by the presence of the P element, a method termed transposon tagging. After the interrupted gene has been cloned, fragments from the mutant allele can be used as a probe to isolate the wild-
Using P elements to insert genes Gerald Rubin and Allan Spradling showed that P-element DNA can be an effective vehicle for transferring donor genes into the germ line of a recipient fly. They devised the following experimental procedure (Figure 15-20). Suppose the goal is to transfer the allele ry+, which confers a characteristic eye color, into the fly genome. The recipient genotype is homozygous for the rosy (ry−) mutation. From this strain, embryos are collected at the completion of about nine nuclear divisions. At this stage, the embryo is one multinucleate cell, and the nuclei destined to form the germ cells are clustered at one end. (P elements mobilize only in germ-
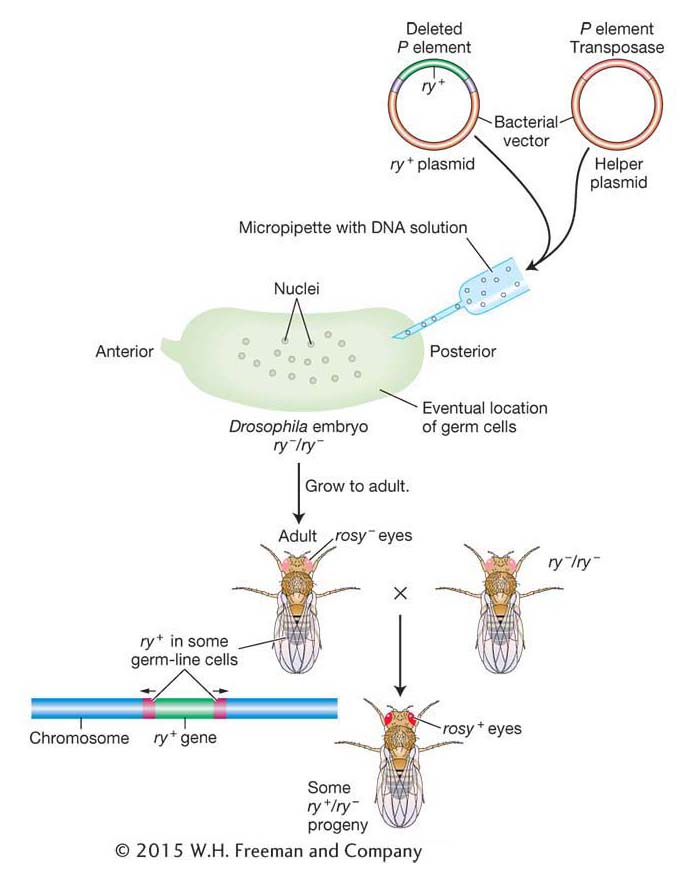
Because the P element can transpose only in Drosophila, these applications are restricted to these flies. In contrast, the maize Ac element is able to transpose after its introduction into the genomes of many plant species, including the mustard weed Arabidopsis, lettuce, carrot, rice, and barley. Like P elements, Ac has been engineered by geneticists for use in gene isolation by transposon tagging. In this way, Ac, the first transposable element discovered by Barbara McClintock, serves as an important tool of plant geneticists more than 50 years later.