4-2 Electrical Activity of a Membrane
Specific aspects of the cell membrane’s electrical activity interact to convey information throughout the nervous system. The movement of ions across neuronal membranes produces the electrical activity that enables this information flow.
Resting Potential
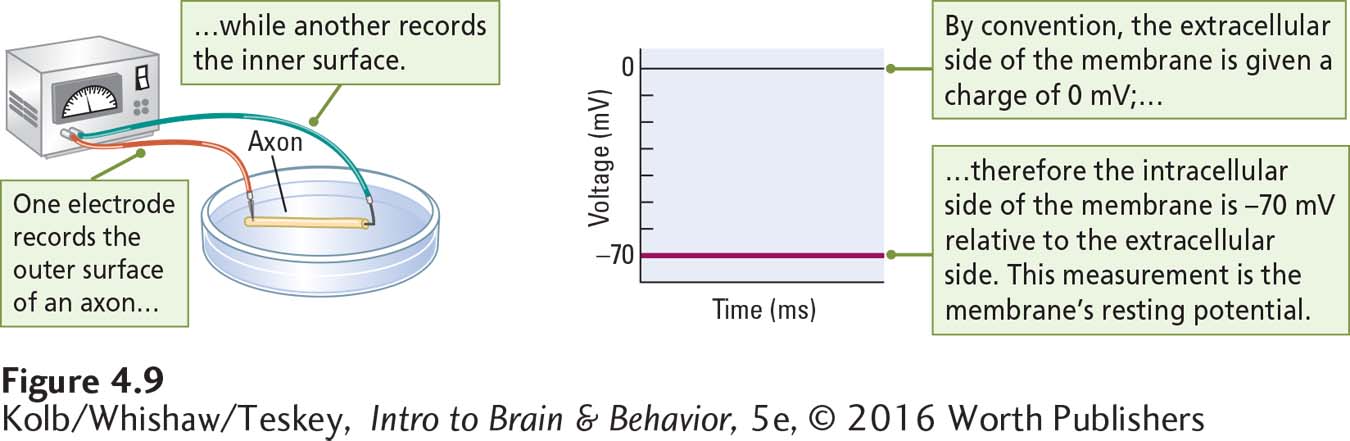
Figure 4-9 shows how the voltage difference is recorded when one microelectrode is placed on the outer surface of an axon’s membrane and another is placed on its inner surface. In the absence of stimulation, the difference is about 70 mV. Although the charge on the outside of the membrane is actually positive, by convention it is given a charge of zero. Therefore, the inside of the membrane at rest is –70 mV relative to the extracellular side.
If we were to continue to record for a long time, the charge across the unstimulated membrane would remain much the same. The charge can change, given certain changes in the membrane, but at rest the difference in charge on the inside and outside of the membrane produces an electrical potential—the ability to use its stored power, analogous to a charged battery. The charge is thus a store of potential energy called the membrane’s resting potential.
We might use the term potential in the same way to talk about the financial potential of someone who has money in the bank—
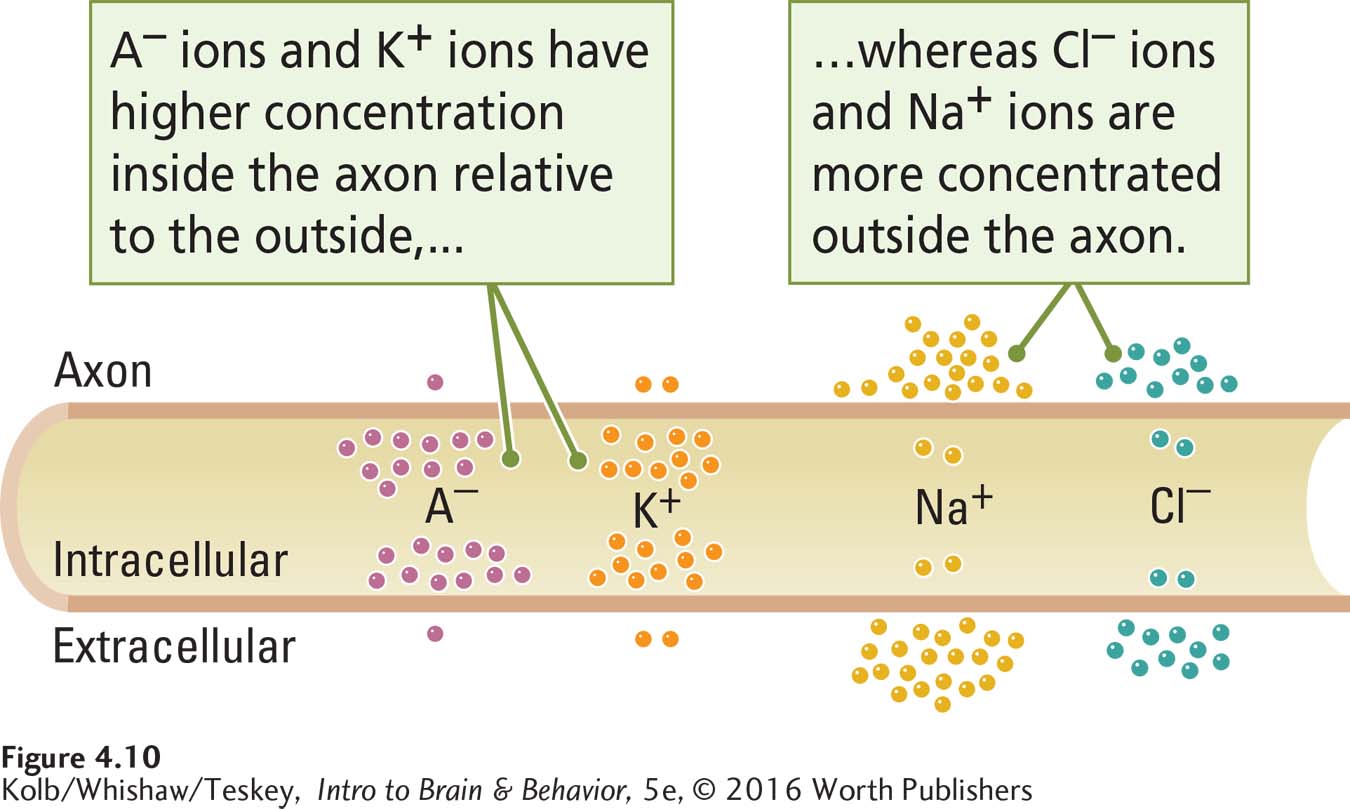
Four charged particles take part in producing the resting potential: ions of sodium (Na+), potassium (K+), chloride (Cl–), and large protein molecules (A–). These are the cations and anions, respectively, defined in Section 4-1. As Figure 4-10 shows, these charged particles are distributed unequally across the axon’s membrane, with more protein anions and potassium ions in the intracellular fluid and more chloride and sodium ions in the extracellular fluid. How do the unequal concentrations arise, and how does each contribute to the resting potential?
Maintaining the Resting Potential
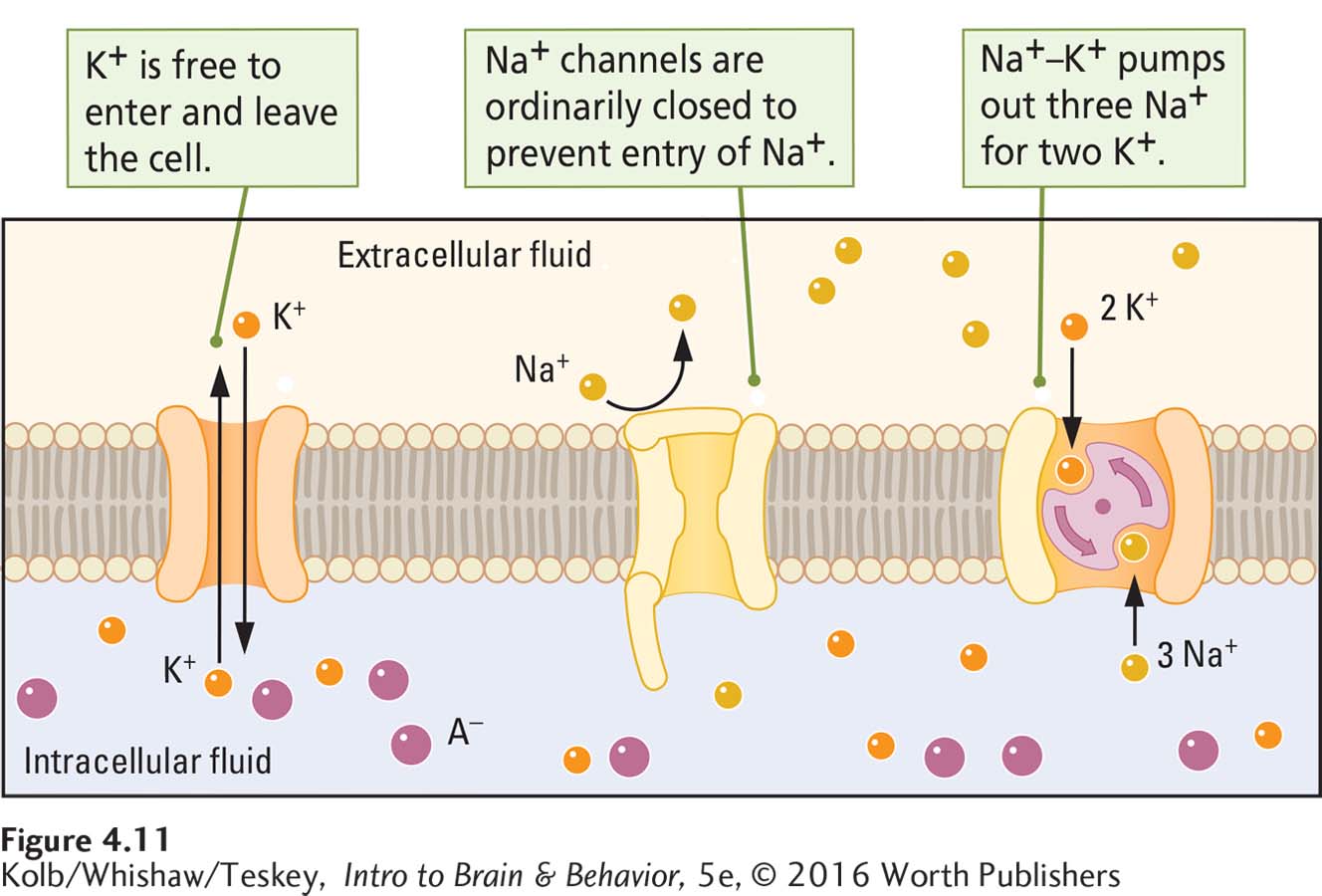
The cell membrane’s channels, gates, and pumps maintain the resting potential. Figure 4-11, which shows the resting membrane close up, details how these three features contribute to the cell membrane’s resting charge:
Because the membrane is relatively impermeable to large molecules, the negatively charged proteins remain inside the cell.
Ungated potassium and chloride channels allow potassium and chloride ions to pass more freely, but gates on sodium channels keep out positively charged sodium ions.
Na+–K+ pumps extrude Na+ from the intracellular fluid and inject K+.
Inside the Cell
Large protein anions are manufactured inside cells. No membrane channels are large enough to allow these proteins to leave the cell, and their negative charge alone is sufficient to produce transmembrane voltage, or a resting potential. Because most cells in the body manufacture these large, negatively charged protein molecules, most cells have a charge across the cell membrane.
To balance the negative charge produced by large protein anions in the intracellular fluid, cells accumulate positively charged potassium ions to the extent that about 20 times as many potassium ions cluster inside the cell as outside it. Potassium ions cross the cell membrane through open potassium channels, as shown in Figure 4-11. With this high concentration of potassium ions inside the cell, however, the potassium concentration gradient across the membrane limits the number of potassium ions entering the cell. In other words, not all the potassium ions that could enter do enter. Because the internal concentration of potassium ions is much higher than the external potassium concentration, potassium ions are drawn out of the cell by the potassium concentration gradient.
A few residual potassium ions on the outside of the membrane are enough to contribute to the charge across the membrane. They add to the negative charge on the intracellular side of the membrane relative to the extracellular side. You may be wondering whether you read the last sentence correctly. If there are 20 times as many potassium ions inside the cell as there are outside, why should the inside of the membrane have a negative charge? Should not all those potassium ions in the intracellular fluid give the inside of the cell a positive charge instead? No, because not quite enough potassium ions are able to enter the cell to balance the negative charge of the protein anions.
Think of it this way: if the number of potassium ions that could accumulate on the intracellular side of the membrane were unrestricted, the positively charged potassium ions inside would exactly match the negative charges on the intracellular protein anions. There would be no charge across the membrane at all. But the number of potassium ions that accumulate inside the cell is limited, because when the intracellular K+ concentration becomes higher than the extracellular concentration, further potassium ion influx is opposed by its concentration gradient.
Outside the Cell
The equilibrium of the potassium voltage and concentration gradients results in some potassium ions remaining outside the cell. It is necessary to have only a few potassium ions outside the cell to maintain a negative charge inside the cell. As a result, potassium ions contribute to the charge across the membrane.
Sodium (Na+) and chloride (Cl–) ions also take part in producing the resting potential. If positively charged sodium ions were free to move across the membrane, they would diffuse into the cell and eliminate the transmembrane charge produced by the unequal distribution of potassium ions inside and outside the cell. This diffusion does not happen, because a gate on the sodium ion channels in the cell membrane is ordinarily closed (see Figure 4-11), blocking the entry of most sodium ions. Still, given enough time, sufficient sodium ions could leak into the cell to neutralize its membrane potential. The cell membrane has a different mechanism to prevent this neutralization.
When sodium ions do leak into the neuron, they are immediately escorted out again by the action of a sodium–
Now consider the chloride ions. Unlike sodium ions, chloride ions move in and out of the cell through open channels in the membrane. The equilibrium point, at which the chloride’s concentration gradient equals its voltage gradient, is approximately the same as the membrane’s resting potential, and so chloride ions ordinarily contribute little to the resting potential. At this equilibrium point, there are about 12 times as many chloride ions outside the cell as inside it.
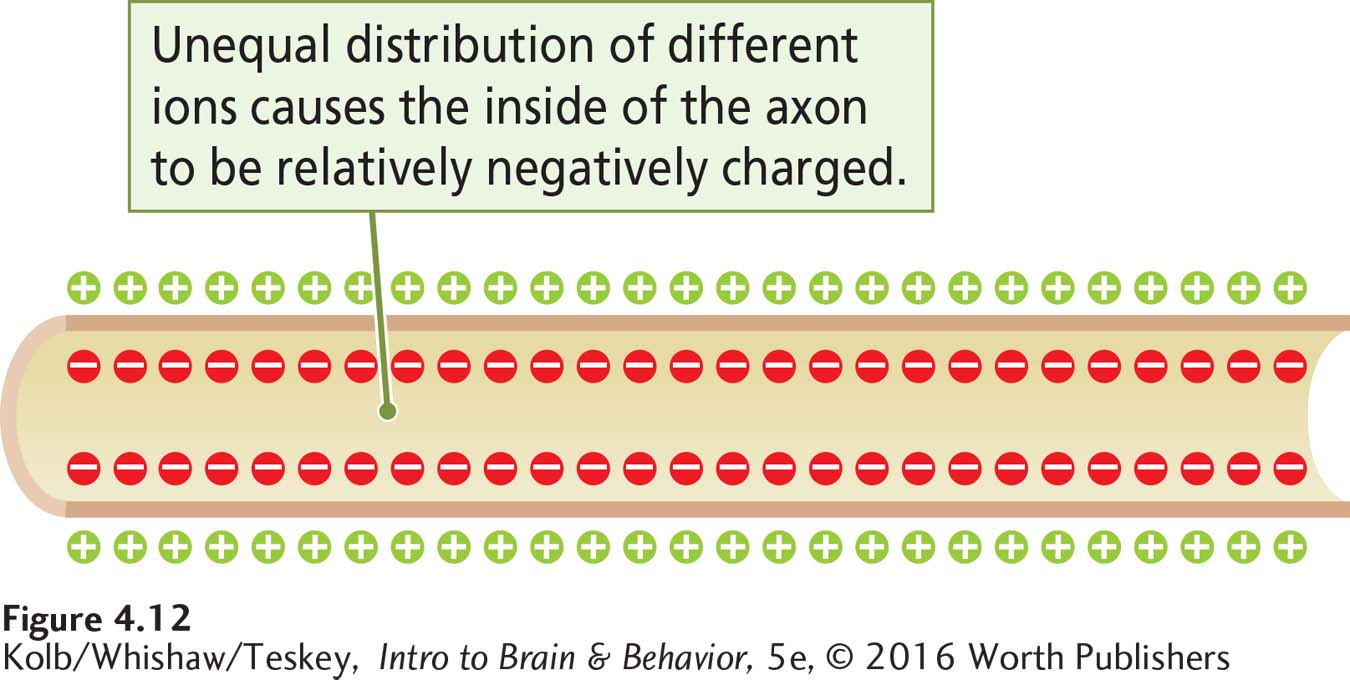
The cell membrane’s semipermeability and the actions of its channels, gates, and pumps thus produce voltage across the cell membrane: its resting potential (Figure 4-12).
Graded Potentials
The resting potential provides an energy store that can be used somewhat like the water in a dam: small amounts can be released by opening gates for irrigation or to generate electricity. If the concentration of any of the ions across the unstimulated cell membrane changes, the membrane voltage changes. These graded potentials are small voltage fluctuations across the cell membrane.
Stimulating a membrane electrically through a microelectrode mimics the way the membrane’s voltage changes to produce a graded potential in the living cell. If the voltage applied to the inside of the membrane is negative, the membrane potential increases in negative charge by a few millivolts. As illustrated in Figure 4-13A, it may change from a resting potential of –70 mV to a slightly greater potential of –73 mV.
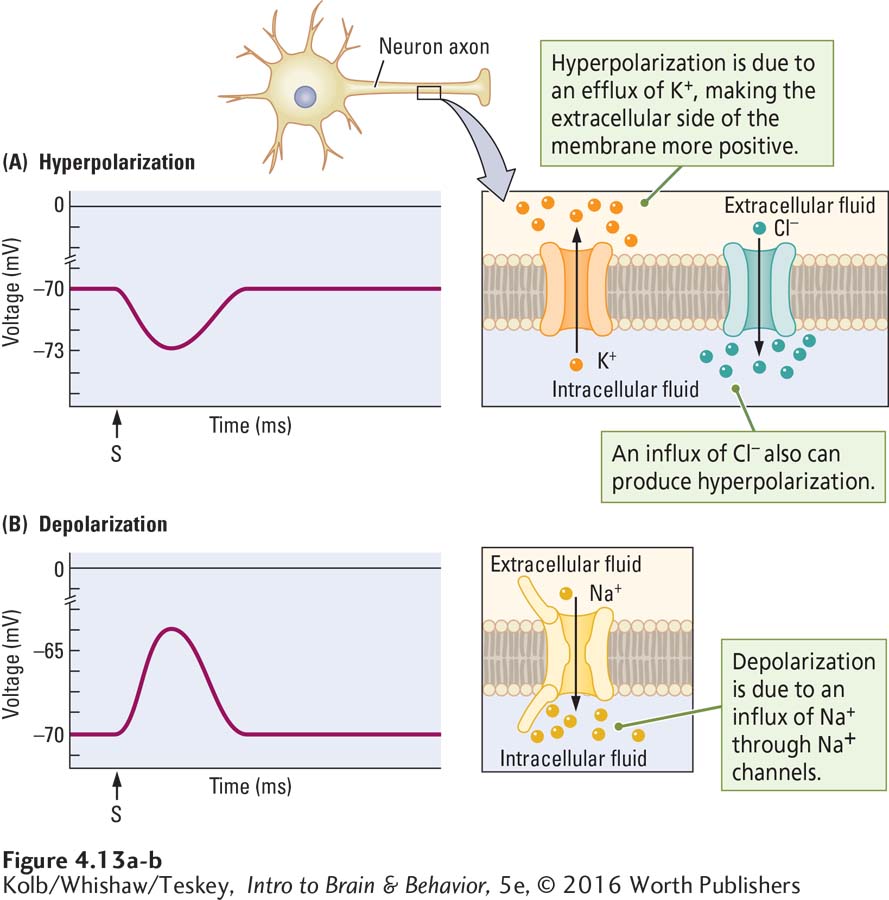
This change is a hyperpolarization because the charge (polarity) of the membrane increases. Conversely, if positive voltage is applied inside the membrane, its potential decreases by a few millivolts. As illustrated in Figure 4-13B, it may change from, say, a resting potential of –70 mV to a slightly lower potential of –65 mV. This change is a depolarization because the membrane charge decreases. Graded potentials usually last only milliseconds.
Hyperpolarization and depolarization typically take place on the soma (cell body) membrane and on neuronal dendrites. These areas contain gated channels that can open and close, changing the membrane potential as illustrated in Figure 4-13. Three channels—

Potassium channels For the membrane to become hyperpolarized, its extracellular side must become more positive, which can be accomplished with an efflux of potassium ions. But if potassium channels are ordinarily open, how can the efflux of potassium ions increase? Apparently, even though potassium channels are open, some resistance to the outward flow of potassium ions remains. Reducing this resistance enables hyperpolarization.
Chloride channels The membrane can also become hyperpolarized if an influx of chloride ions occurs. Even though chloride ions can pass through the membrane, more ions remain on the outside than on the inside, so a decreased resistance to Cl– flow can result in brief increases of Cl– inside the cell.
Sodium channels Depolarization can be produced if normally closed sodium channel gates open to allow an influx of sodium ions.
Evidence that potassium channels have a role in hyperpolarization comes from the fact that the chemical tetraethylammonium (TEA), which blocks potassium channels, also blocks hyperpolarization. The involvement of sodium channels in depolarization is indicated by the fact that the chemical tetrodotoxin (TTX), which blocks sodium channels, also blocks depolarization. The puffer fish, considered a delicacy in some countries, especially Japan, secretes this potentially deadly poison to fend off potential predators. Skill is required to prepare this fish for dinner. It can be lethal to the guests of careless cooks because its toxin impedes the electrical activity of neurons.
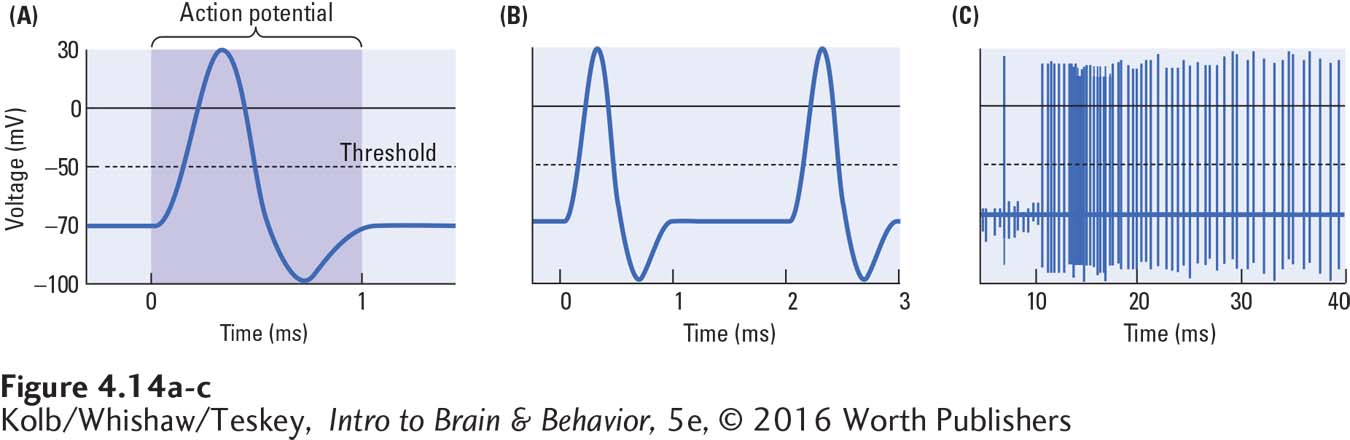
Action Potential
Electrical stimulation of the cell membrane at resting potential produces local graded potentials. An action potential is a brief but large reversal in an axon membrane’s polarity (Figure 4-14A). It lasts about 1 ms. The voltage across the membrane suddenly reverses, making the intracellular side positive relative to the extracellular side, then abruptly reverses again to restore the resting potential. Because the action potential is brief, many action potentials can occur within a second, as illustrated in Figure 4-14B and C, where the time scales are compressed.
An action potential occurs when a large concentration of first Na+ and then K+ crosses the membrane rapidly. The depolarizing phase of the action potential is due to Na+ influx, and the hyperpolarizing phase, to K+ efflux. Sodium rushes in, then potassium rushes out. As shown in Figure 4-15, the combined flow of sodium and potassium ions underlies the action potential.
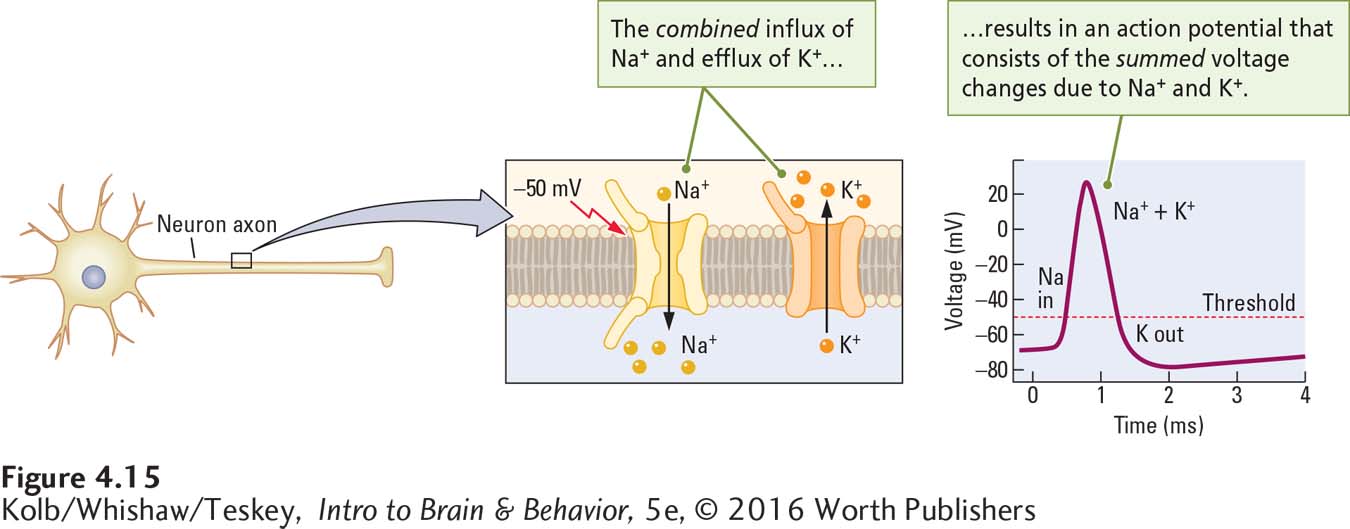
An action potential is triggered when the cell membrane is depolarized to about –50 mV. At this threshold potential, the membrane charge undergoes a remarkable further change with no additional stimulation. The relative voltage of the membrane drops to zero and continues to depolarize until the charge on the inside of the membrane is as great as +30 mV—
The action potential normally consists of the summed current changes caused first by the inflow of sodium and then by the outflow of potassium on an axon. Experimental results reveal that if an axon membrane is stimulated electrically while the solution surrounding the axon contains the chemical TEA (to block potassium channels), the result is a smaller-
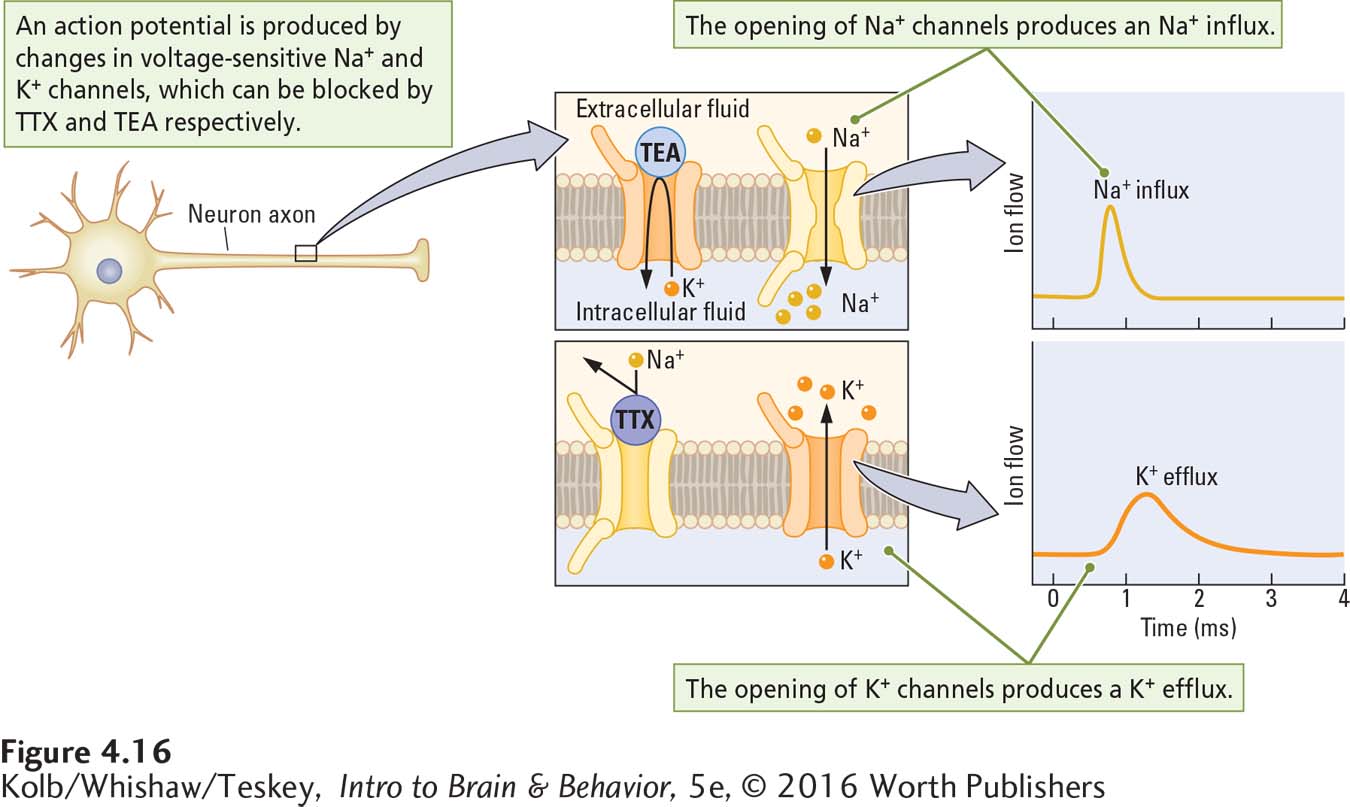
Role of Voltage-
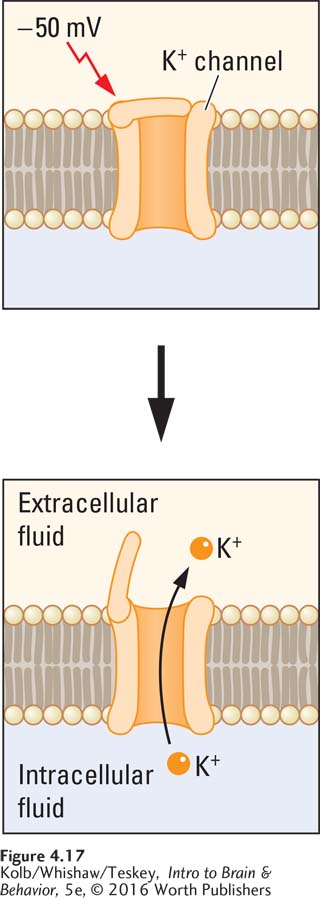
What cellular mechanisms underlie the movement of sodium and potassium ions to produce an action potential? The answer is the behavior of a class of gated sodium and potassium channels sensitive to the membrane’s voltage (Figure 4-17). These voltage-
Both sodium and potassium voltage-
sensitive channels are attuned to the threshold voltage of about –50 mV. If the cell membrane changes to reach this voltage, both types of channels open to allow ion flow across the membrane. The voltage-
sensitive sodium channels are more sensitive than the potassium channels and so open first. As a result, the voltage change due to Na+ influx takes place slightly before the voltage change due to K+ efflux can begin. Sodium channels have two gates. Once the membrane depolarizes to about +30 mV, one of the gates closes. Thus, Na+ influx begins quickly and ends quickly.
The potassium channels open more slowly than the sodium channels, and they remain open longer. Thus, the efflux of K+ reverses the depolarization produced by Na+ influx and even hyperpolarizes the membrane.
Action Potentials and Refractory Periods
There is an upper limit to how frequently action potentials occur, and sodium and potassium channels are responsible for it. Stimulation of the axon membrane during the depolarizing phase of the action potential will not produce another action potential. Nor is the axon able to produce another action potential when it is repolarizing. During these times, the membrane is described as being absolutely refractory.
If on the other hand the axon membrane is stimulated during hyperpolarization, another action potential can be induced, but the second stimulation must be more intense than the first. During this phase, the membrane is relatively refractory.
Exceptions do exist: some CNS neurons discharge during the repolarizing phase.
Refractory periods result from the way gates of the voltage-
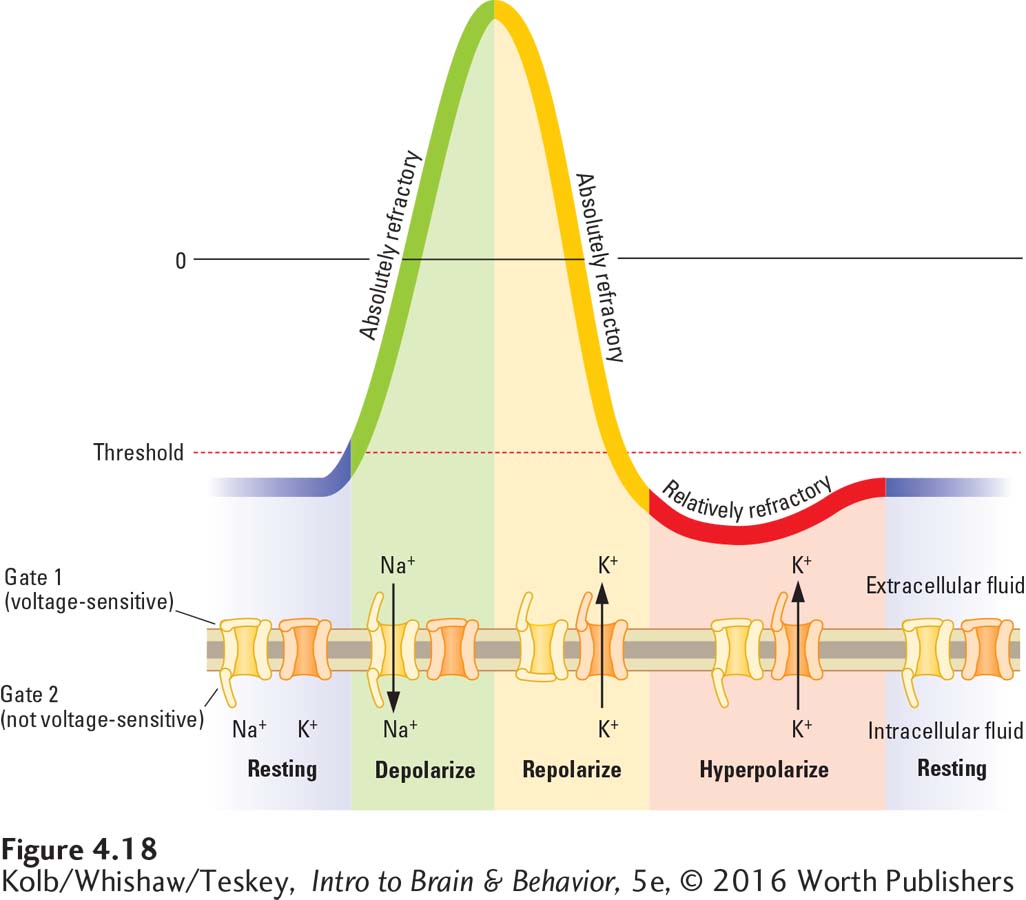
During the resting potential, gate 1 of the sodium channel depicted in Figure 4-18 is closed; only gate 2 is open. At the threshold level of stimulation, gate 1 also opens. Gate 2, however, closes very quickly after gate 1 opens. This sequence produces a brief period during which both sodium gates are open. When both gates are open and when gate 2 is closed, the membrane is absolutely refractory.
The opening of the potassium channels repolarizes and eventually hyperpolarizes the cell membrane. The potassium channels open and close more slowly than the sodium channels do. The hyperpolarization produced by a continuing efflux of potassium ions makes it more difficult to depolarize the membrane to the threshold that reopens the gates underlying an action potential. While the membrane is hyperpolarizing, it is relatively refractory.
The action of a lever-
During the flush, the toilet is absolutely refractory: another flush cannot be induced at this time. During the refilling of the bowl, in contrast, the toilet is relatively refractory, meaning that flushing again is possible but harder. Only after the cycle is over and the toilet is once again at rest can a full flush be produced again.
Nerve Impulse
Suppose you place two recording electrodes at a distance from one another on an axon membrane, then electrically stimulate an area adjacent to one electrode. That electrode would immediately record an action potential. A similar recording would register on the second electrode in a flash. An action potential has arisen near this second electrode also, even though it is some distance from the original point of stimulation.
Is this second action potential simply an echo of the first that passes down the axon? No, it cannot be, because the action potential’s size and shape are exactly the same at the two electrodes. The second is not just a faint, degraded version of the first but is equal in magnitude. Somehow the full action potential has moved along the axon. This propagation of an action potential along an axon is called a nerve impulse.
Why does an action potential move? Remember that the total voltage change during an action potential is 100 mV, far beyond the 20-
When the membrane at an adjacent part of the axon reaches –50 mV, the voltage-
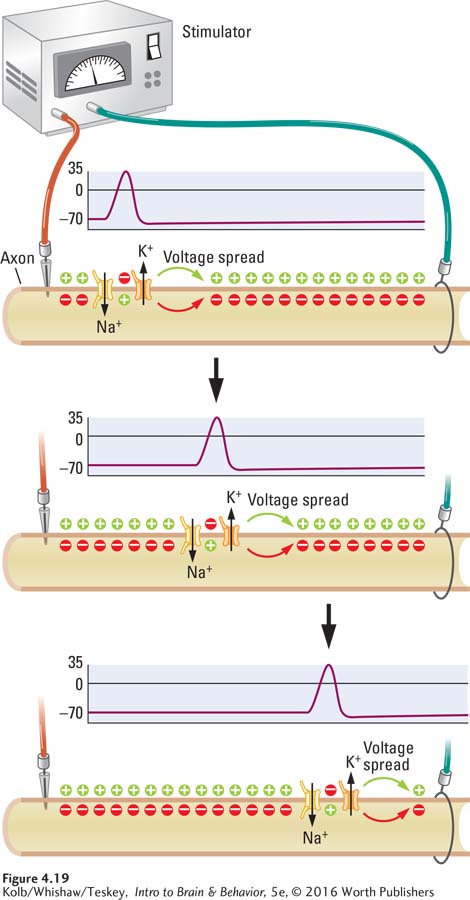
Because they are propagated by gated ion channels acting on the membrane in their own vicinity, action potentials on a nerve or tract are the same magnitude wherever they occur. An action potential depends on energy expended where it occurs, and the same amount of energy is expended at every site along the membrane as a nerve impulse is propagated.

As a result, action potentials do not dissipate: an action potential is either generated completely or not generated at all. Action potentials are all-
Think of the voltage-
Essentially, the domino effect happens when voltage-
Refractory Periods and Nerve Action
Refractory periods are determined by the position of the gates that mediate ion flow in the voltage-
First, the maximum rate at which action potentials can occur is about 200 per second (1 s or 1000 ms/5 ms limit = 200 action potentials in 1 s). The sensitivity of voltage-
Second, although an action potential can travel in either direction on an axon, refractory periods prevent it from reversing direction and returning to its point of origin. Refractory periods thus produce a single, discrete impulse that travels away from the initial point of stimulation. When an action potential begins near the cell body, it usually travels down the axon to the terminals.
To return to our domino analogy, once a domino falls, setting it back up takes time. This is its refractory period. Because each domino falls as it knocks down its neighbor, the sequence cannot reverse until the domino is set upright again: the dominos can fall in only one direction. The same principle determines the action potential’s direction.
Saltatory Conduction and the Myelin Sheath
Because the giant axons of squid are so large, they can transmit nerve impulses very quickly, much as a large-
Our largest axons are only about 30 µm wide, so the speed with which they convey information should not be especially fast. And yet, like most vertebrate species, we humans are hardly sluggish creatures. We process information and generate responses with impressive speed. How do we manage to do so if our axons are so thin? The vertebrate nervous system has evolved a solution that has nothing to do with axon size.
Glial cells play a role in speeding nerve impulses in the vertebrate nervous system. Schwann cells in the human peripheral nervous system and oligodendroglia in the central nervous system wrap around each axon, forming the myelin sheath that insulates it (Figure 4-20). Action potentials cannot occur where myelin is wrapped around an axon. For one thing, the myelin is an insulating barrier to ionic current flow. For another, axonal regions that lie under myelin have few channels through which ions can flow, and ion channels are essential to generating an action potential.
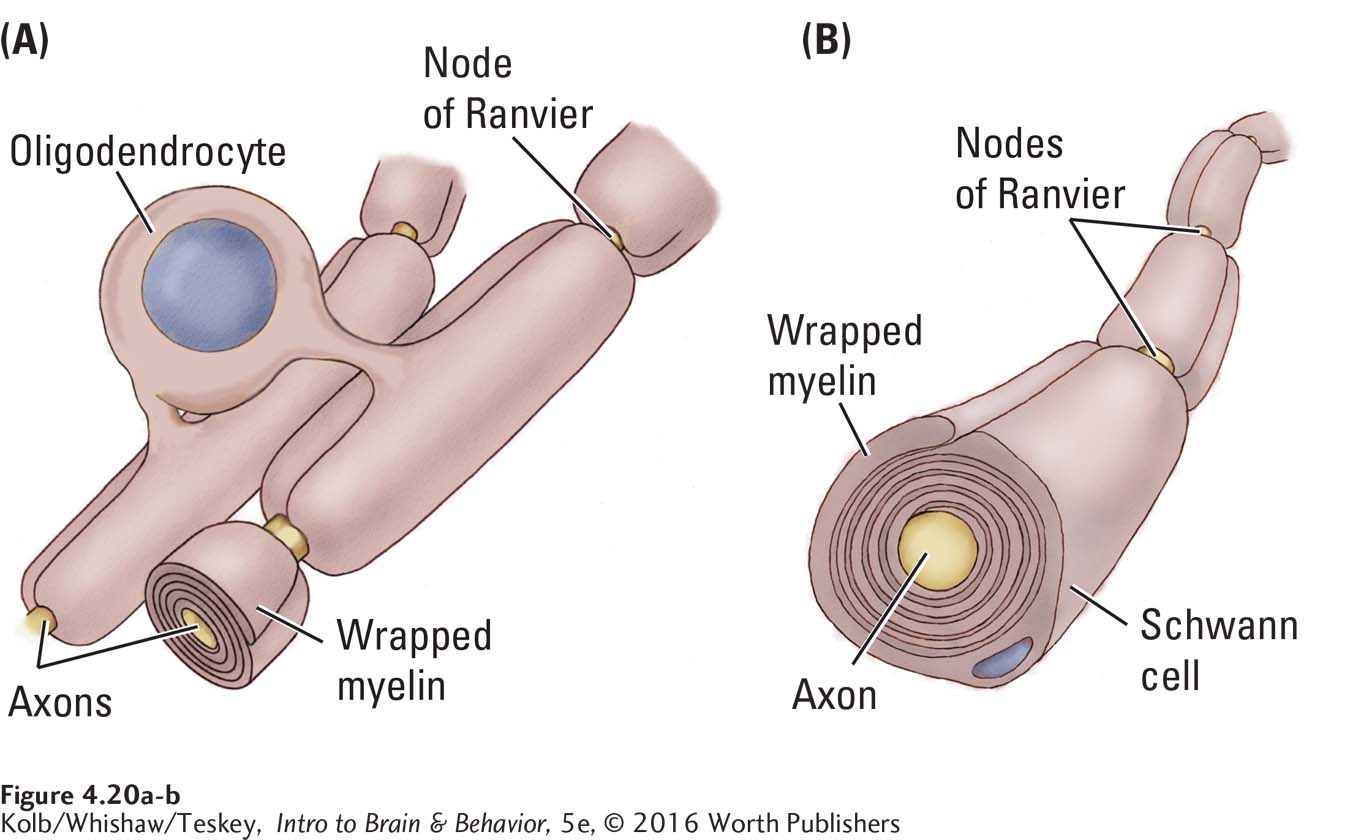
To review glial cell types, appearance, and functions, see Table 3-1.
But axons are not totally encased in myelin. Unmyelinated gaps between successive glial cells are richly endowed with voltage-
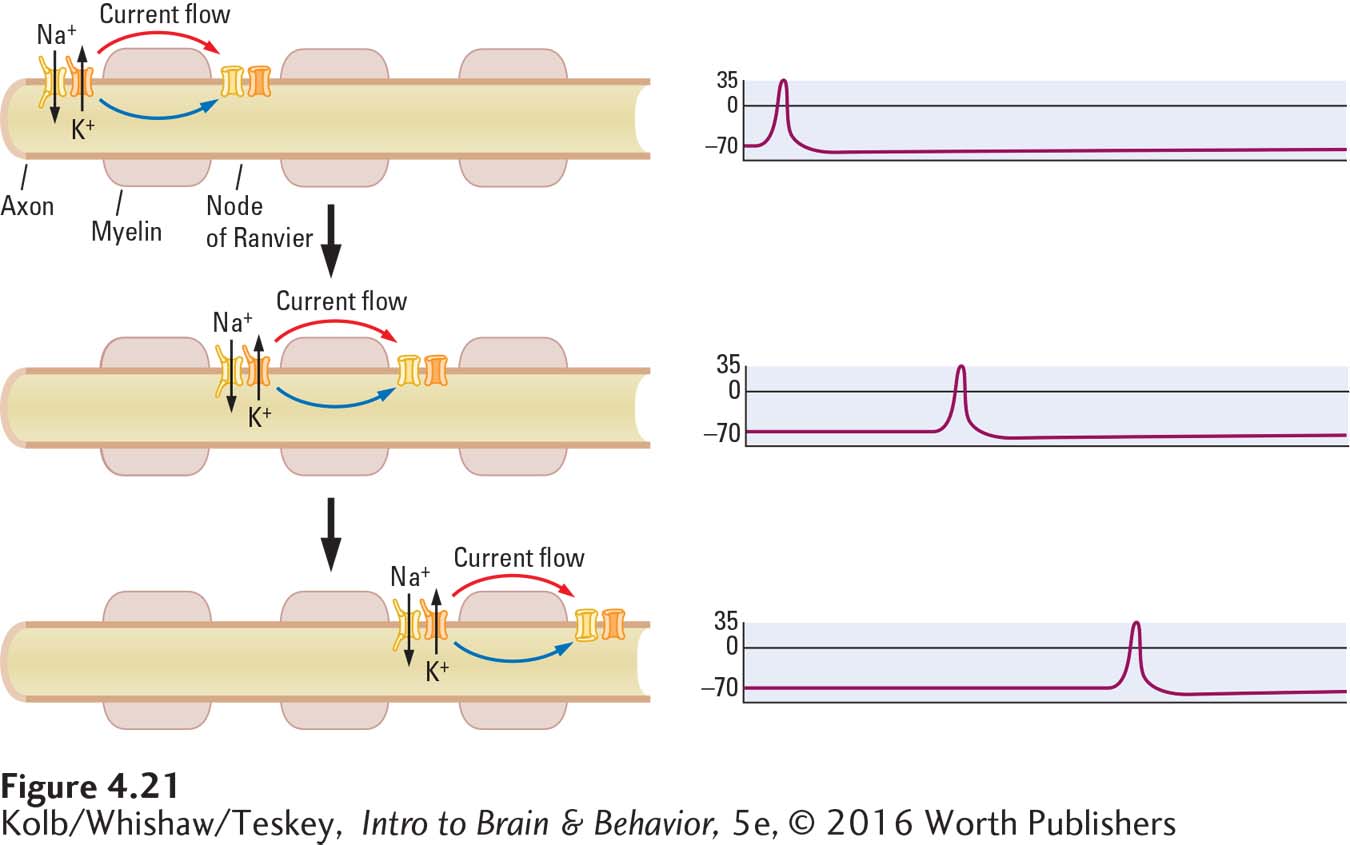
Myelin has two important consequences for propagating action potentials. First, propagation becomes energetically cheaper, since action potentials regenerate only at the nodes of Ranvier, not along the axon’s entire length. Action potential conduction in unmyelinated axons, by contrast, has a significant metabolic energy cost (Crotty et al., 2006). The second consequence is that myelin improves the action potential’s conduction speed.
Jumping from node to node speeds the rate at which an action potential can travel along an axon, because the current flowing within the axon beneath the myelin sheath travels very fast. While the current moves speedily, the voltage drops quickly over distance. But the nodes of Ranvier are spaced ideally to ensure sufficient voltage at the next node to regenerate the action potential. On larger, myelinated mammalian axons, nerve impulses can travel at a rate as high as 120 meters per second. On smaller, uninsulated axons they travel only about 30 meters per second.
Spectators at sporting events sometimes initiate a wave that travels around a stadium. Just as one person rises, the next person begins to rise, producing the wave effect. This human wave is like conduction along an unmyelinated axon. Now think of how much faster the wave would complete its circuit around the field if only spectators in the corners rose to produce it. This is analogous to a nerve impulse that travels by jumping from one node of Ranvier to the next. The quick reactions that humans and other mammals are capable of are due in part to this saltatory conduction in their nervous system.
Neurons that send messages over long distances quickly, including sensory and motor neurons, are heavily myelinated. If myelin is damaged, a neuron may be unable to send any messages over its axons. In multiple sclerosis (MS), the myelin formed by oligodendroglia is damaged, which disrupts the functioning of neurons whose axons it encases. Clinical Focus 4-2, Multiple Sclerosis on page 126, describes the course of the disease.
4-2
Multiple Sclerosis
One day J. O., who had just finished university requirements to begin work as an accountant, noticed a slight cloudiness in her right eye. It did not go away when she wiped her eye. Rather, the area grew over the next few days. Her optometrist suggested that she see a neurologist, who diagnosed optic neuritis, an indication that can be a flag for multiple sclerosis (MS).
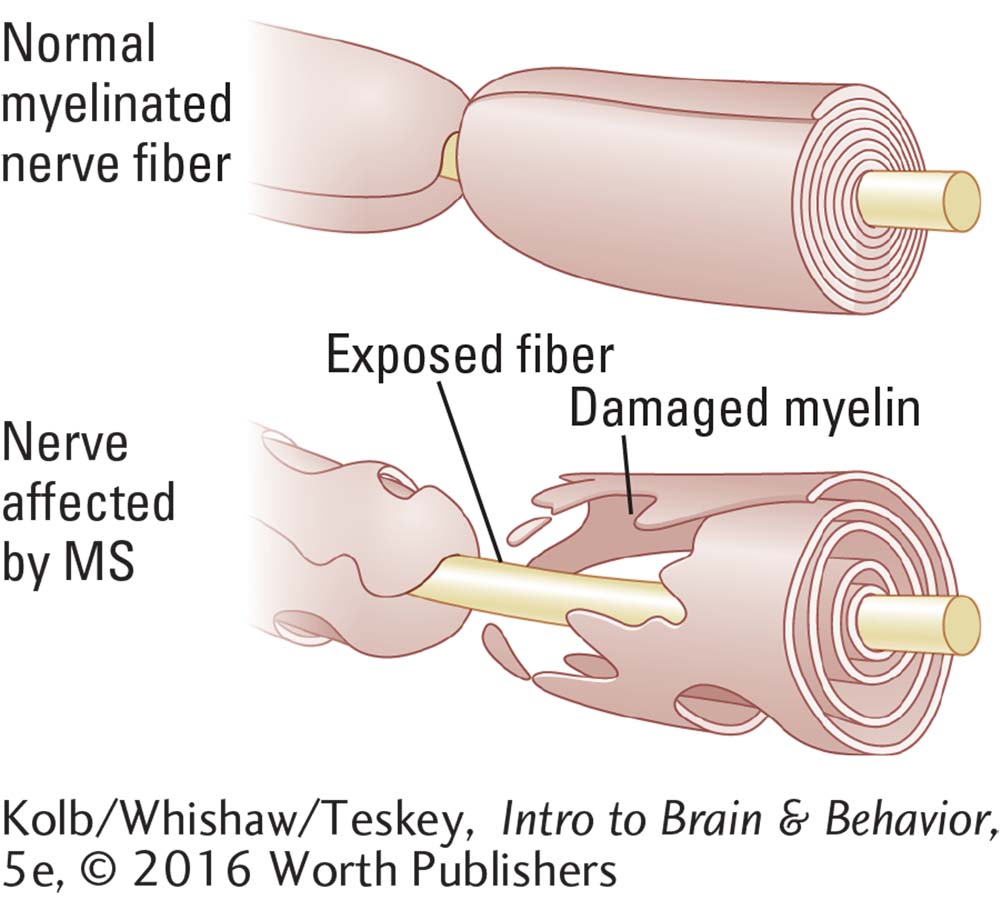
MS is caused by a loss of myelin produced by oligodendroglia cells in the CNS (see illustration). It disrupts the affected neurons’ ability to propagate action potentials via saltatory conduction. This loss of myelin occurs in patches, and scarring frequently results in the affected areas.
Eventually, a hard scar, or plaque, forms at the site of myelin loss. (MS is called a sclerosis from the Greek word meaning hardness.) Associated with the loss of myelin is impairment of neuron function, causing characteristic MS symptoms of sensory loss and difficulty in moving.
Fatigue, pain, and depression are commonly associated with MS. Bladder dysfunction, constipation, and sexual dysfunction all complicate it. MS, about twice as common in women as in men, greatly affects a person’s emotional, social, and vocational functioning.
Multiple sclerosis is the most common of nearly 80 autoimmune diseases, conditions in which the immune system makes antibodies to a person’s own body (Rezania et al., 2012). Although MS patients are treated with anti-
J. O.’s eye cleared over the next few months, and she had no further symptoms until after the birth of her first child 3 years later, when she felt a tingling in her right hand. The tingling spread up her arm, until gradually she lost movement in the arm for 5 months. Then J. O.’s arm movement returned. But 5 years later, after her second child was born, she felt a tingling in her left big toe that spread along the sole of her foot and then up her leg, eventually leading again to loss of movement. J. O. received corticosteroid treatment, which helped, but the condition rebounded when she stopped treatment. Then it subsided and eventually disappeared.
Since then, J. O. has had no major outbreaks of motor impairment, but she reports enormous fatigue, takes long naps daily, and is ready for bed early in the evening. Her sister and a female cousin have experienced similar symptoms, and recently a third sister began to display similar symptoms in middle age. One of J. O.’s grandmothers was confined to a wheelchair, although the source of her problem was never diagnosed.
MS is difficult to diagnose. Symptoms usually appear in adulthood, their onset is quite sudden and their effects can be swift. Initial symptoms may be loss of sensation in the face, limbs, or body or loss of control over movements or loss of both sensation and control. Motor symptoms usually appear first in the hands or feet.
Early symptoms often go into remission and do not appear again for years. In some forms, however, MS progresses rapidly over just a few years until the person is bedridden.
MS is common in the most northern and most southern latitudes, so it may be related to a lack of vitamin D, which is produced by the action of sunlight on the skin. The disease may also be related to genetic susceptibility, as is likely in J. O.’s case. Many MS patients take vitamin D3 and vitamin B12.
It has been suggested that blood flow from the brain is reduced in MS, allowing a buildup of toxic iron. Widening veins that drain blood from the brain is suggested as a treatment. Clinical trials have been initiated on the basis of reports from media and in response to patient groups rather than on established scientific evidence. It has been argued that methodological flaws and lack of evidence disqualify both the venous cause of MS and venous widening as an appropriate treatment (Valdueza et al., 2013).
4-2 REVIEW
Electrical Activity of a Membrane
Before you continue, check your understanding.
Question 1
The _________ results from the unequal distribution of _______ inside and outside the cell membrane.
Question 2
Because it is _________, the cell membrane prevents the efflux of large protein anions and pumps sodium ions out of the cell to maintain a slightly _______ charge in the intracellular fluid relative to the extracellular fluid.
Question 3
For a graded potential to arise, a membrane must be stimulated to the point that the transmembrane charge increases slightly to cause a(n) ________ or decreases slightly to cause a(n) ______.
Question 4
The voltage change associated with a(n) _________ is sufficiently large to stimulate adjacent parts of the axon membrane to the threshold for propagating it along the length of an axon as a(n) _________.
Question 5
Briefly explain why nerve impulses travel faster on myelinated than on unmyelinated axons.
Answers appear in the Self Test section of the book.