4-1 Searching for Electrical Activity in the Nervous System
The first hints about how the nervous system conveys its messages came in the eighteenth century, following the discovery of electricity. Early discoveries about the nature of electricity quickly led to proposals that it plays a role in conducting information in the nervous system. We describe a few milestones that led from this idea to an understanding of how the nervous system really conveys information. If you have a basic understanding of how electricity works and how it is used to stimulate neural tissue, read on. If you prefer to brush up on electricity and electrical stimulation first, turn to The Basics: Electricity and Electrical Stimulation on page 110.
Early Clues That Linked Electricity and Neuronal Activity
In a dramatic demonstration in 1731, Stephen Gray, an amateur English scientist, rubbed a rod with a piece of cloth to accumulate electrons on the rod. Then he touched the charged rod to the feet of a boy suspended on a rope and brought a metal foil to the boy’s nose. The foil was attracted to the boy’s nose and bent on approaching it, and as foil and nose touched, electricity passed from the rod through the boy to the foil.
Yet the boy felt nothing. Therefore, Gray speculated that electricity might be the messenger that spreads information through the nervous system. Two other lines of evidence, drawn from electrical stimulation and electrical recording studies, implicated electrical activity in the nervous system’s flow of information.
Gray’s experiment resembles accumulating electrons by combing your hair. Hold a piece of paper near the comb, and the paper bends toward it. Negative charges on the comb push negative charges on the paper to its backside, leaving the front side positively charged. Because opposite charges attract, the paper bends toward the comb.
Electrical Stimulation Studies
When the Italian scientist Luigi Galvani, a contemporary of Gray, observed that frogs’ legs hanging on a wire in a market twitched during a lightning storm, he surmised that sparks of electricity from the storm were activating the leg muscles. Investigating this possibility, he found that if an electrical current is applied to a dissected nerve, the muscle connected to that nerve contracts. While it was unclear how the process worked, Galvani had discovered electrical stimulation: passing an electrical current from the uninsulated tip of an electrode onto a nerve to produce behavior—
Among the many researchers who used Galvani’s technique to produce muscle contraction, two mid-
THE BASICS
Electricity and Electrical Stimulation
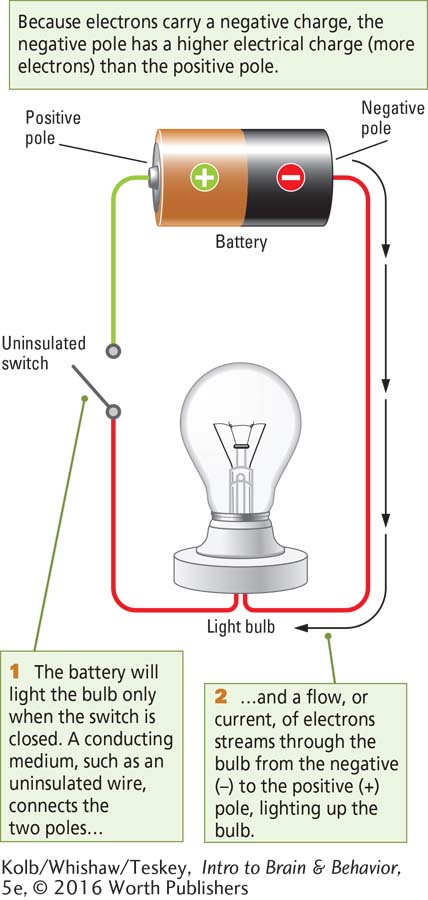
Electricity powers the lights in your home and the batteries that run so many electronic gadgets, from smartphones to electric cars. Electricity is the flow of electrons from a body that contains a higher charge (more electrons) to a body that contains a lower charge (fewer electrons). This electron flow can perform work—
How Electricity Works
In Power Source, negatively charged electrons are attracted to the positive pole because opposite charges attract. The electrons on the negative pole have the potential to flow to the positive pole. This electrical potential, or electrical charge, is the ability to do work using stored electrical energy.
Electrical potential is measured in volts, the difference in charge between the positive and the negative poles. These poles are separated by an insulator. Thus, when not connected, the positive and negative poles in a battery, like the poles in each wall socket in your home, hold voltage between the poles.
Electrical Activity in Cells
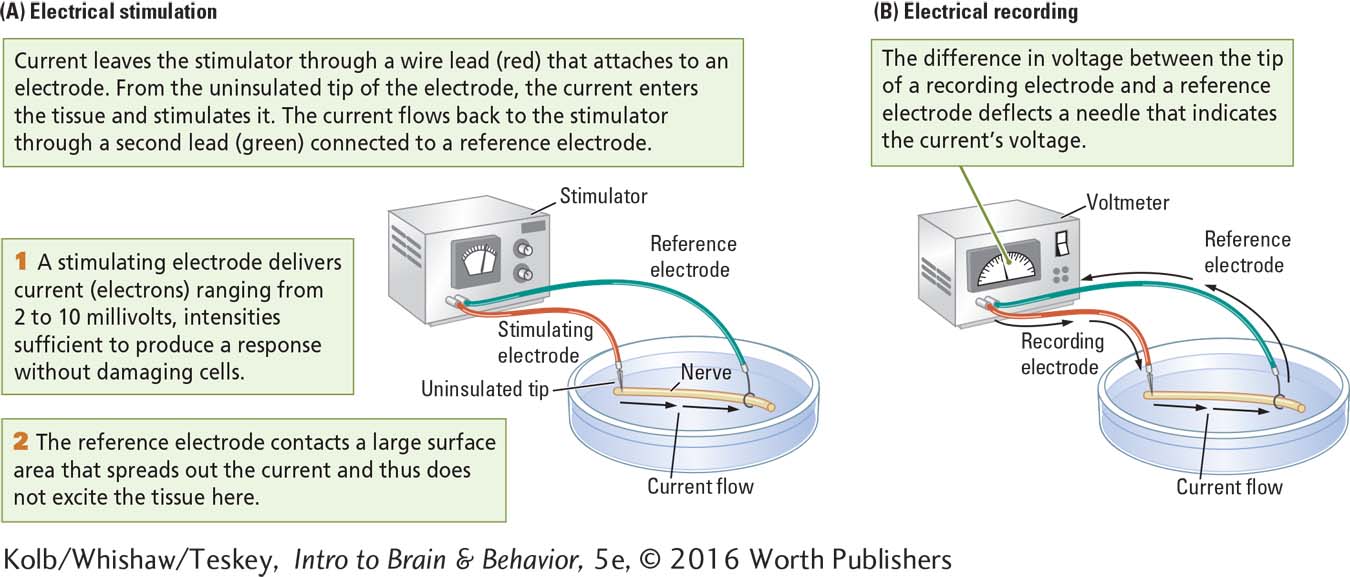
If the bare tip of an insulated wire, or electrode, from each pole of a battery comes into contact with biological tissue, current will flow from the electrode connected to the negative pole into the tissue and from the tissue into the electrode connected to the positive pole. The stimulation comes from the electrode’s uninsulated tip. Microelectrodes can record from or stimulate tissue as small as parts of a single living cell.
Electrical stimulation, illustrated in part A of Studying Electrical Activity in Animal Tissue, is most effective when administered in brief pulses. A timer in the stimulator turns the current on and off to produce the pulses. In electrical recording, voltage can be displayed by the dial on a voltmeter, a recording device that measures the voltage of a battery or of biological tissue (part B).
In 1874, Roberts Bartholow, a Cincinnati physician, first described the effects of human brain stimulation. His patient, Mary Rafferty, had a skull defect that exposed part of her neocortex. Bartholow stimulated her exposed brain tissue to examine the effects. In one of his observations he wrote:
Passed an insulated needle into the left posterior lobe so that the non-
By the 1960s, the scientific community had established ethical standards for research on human and nonhuman subjects (see Section 7-7). Today, brain stimulation is standard in many neurosurgical procedures (see Section 16-2).
As you might imagine, Bartholow’s report was not well received! The uproar after its publication forced him to leave Cincinnati. Despite the questionable ethics of his experiment, Bartholow had demonstrated that the brain of a conscious person could be stimulated electrically to produce movement of the body.
Electrical Recording Studies
A less invasive line of evidence that information flow in the brain is partly electrical came from the results of electrical recording experiments. Richard Caton, a Scottish physician who lived a century ago, was the first to measure the brain’s electrical currents with a sensitive voltmeter, a device that measures the flow and the strength of electrical voltage by recording the difference in electrical potential between two bodies. When he placed electrodes on a human subject’s skull, Caton reported fluctuations in his voltmeter recordings. Today, this type of brain recording, the electroencephalogram (EEG), is a standard tool used, among other things, to monitor sleep stages and to detect the excessive neural synchrony that characterizes electrographic seizures, as described in Clinical Focus 4-1, Epilepsy.
Detail on these EEG applications appears in Sections 7-2, 13-3, and 16-3.
These pioneering studies provided evidence that neurons send electrical messages, but concluding that nerves and tracts carry the kind of electrical current that powers your phone would be incorrect. Hermann von Helmholtz, a nineteenth-
Information flow in the nervous system, then, is much too slow to be a flow of electricity (based on electrons). To explain the electrical signals of a neuron, Julius Bernstein suggested in 1886 that neuronal chemistry (based on ions) produces an electrical charge. He also proposed that the charge can change and so act as a signal. Bernstein’s idea was that successive waves of electrical change constitute the message conveyed by the neuron.
Moreover, it is not the ions themselves that travel along the axon but rather a wave of charge. To understand the difference, consider other kinds of waves. If you drop a stone into a pool of still water, the contact produces a wave that travels away from the site of impact, as shown in Figure 4-2. The water itself does not travel. Only the change in pressure moves, shifting the height of the water surface and producing the wave effect.
Similarly, when you speak, you induce pressure waves in air, and these waves carry the sound of your voice to a listener. If you flick a towel, a wave travels to the other end of the towel. Just as waves through the air send a spoken message, Bernstein’s idea was that waves of chemical change travel along an axon to deliver a neuron’s message.

Tools for Measuring a Neuron’s Electrical Activity
Waves that carry nervous system messages are minute and are restricted to the surfaces of neurons. Still, we can produce these waves using conventional electrical stimulation and measure them using electrical recording techniques to determine how they are produced. When a single axon is stimulated, it produces a wave of excitation. If an electrode connected to a voltmeter is placed on a single axon, as illustrated in Figure 4-3, the electrode can detect a change in electrical charge on that axon’s membrane as the wave passes.
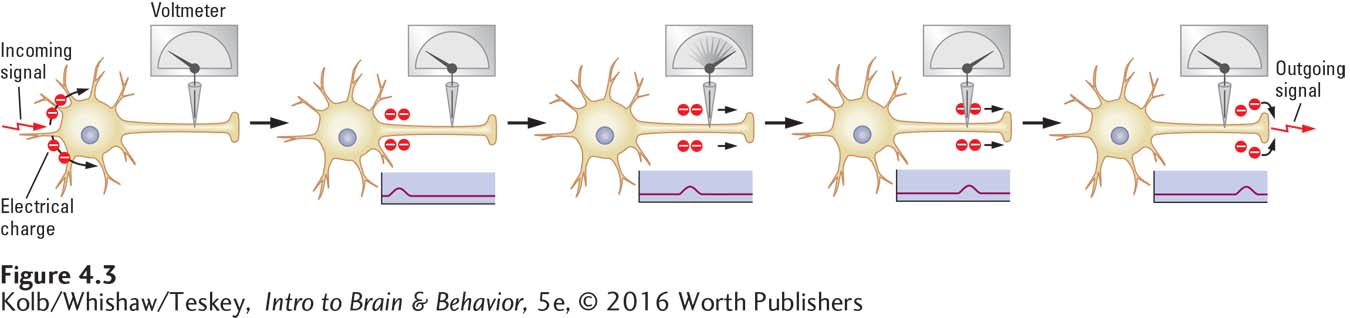
As simple as this process may seem, recording a wave and explaining how it is produced require a neuron large enough to record, a recording device sensitive enough to detect a tiny electrical impulse, and an electrode small enough to be placed on the surface of a single neuron. The fortuitous discovery of the giant axon of the squid, the invention of the oscilloscope, and the development of microelectrodes met all these requirements.
Giant Axon of the Squid
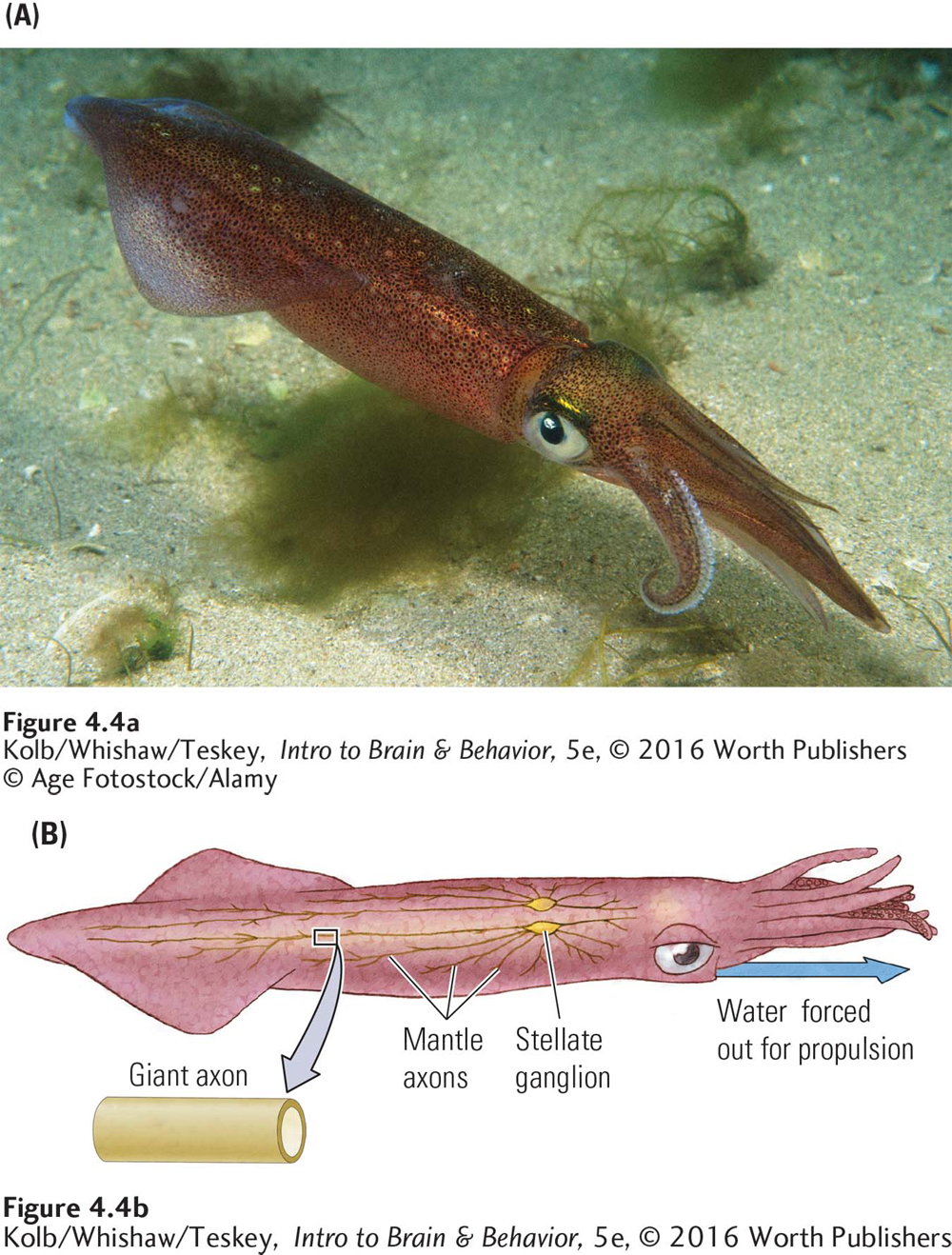
The neurons of most animals, including humans, are tiny, on the order of 1 to 20 micrometers (µm) in diameter, too small to be seen by the naked eye. The British zoologist J. Z. Young, when dissecting the North Atlantic squid, Loligo vulgaris, noticed that it has giant axons, as much as a millimeter (1000 µm, or about a twenty-
1 micron (µm) = one-
Loligo is not a giant squid. It is only about a foot long. But its axons are giant, as axons go. Each is formed by the fusion of many smaller axons. Because larger axons send messages faster than smaller axons do, these giant axons allow the squid to jet-
In 1936, Young suggested to Alan Hodgkin and Andrew Huxley, neuroscientists at Cambridge University in England, that Loligo’s axons were large enough to be used for electrical recording studies. A giant axon could be dissected out of the squid and kept functional in a bath of salty liquid that approximates body fluids. In this way, Hodgkin and Huxley (1939) described the neuron’s electrical activity. In 1963 they received the Nobel Prize for their accomplishment.
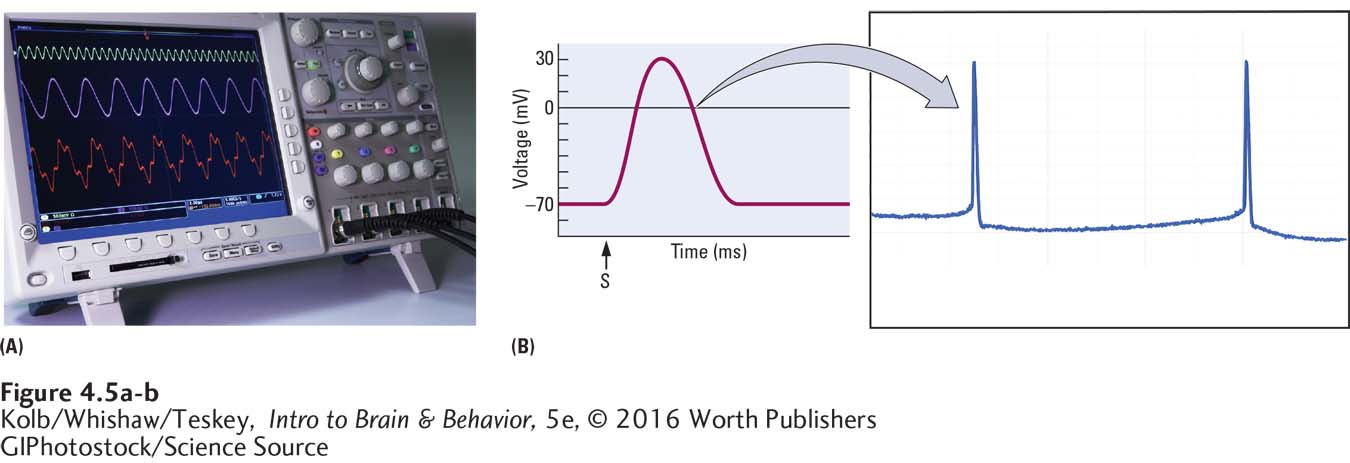
Oscilloscope
Hodgkin and Huxley’s experiments were made possible by the invention of the oscilloscope, a voltmeter with a screen sensitive enough to display the minuscule electrical signals emanating from a nerve or neuron over time (Figure 4-5A). As graphed in Figure 4-5B, the units used when recording the electrical charge from a nerve or neuron are millivolts (mV; 1 mV is one-
Microelectrodes
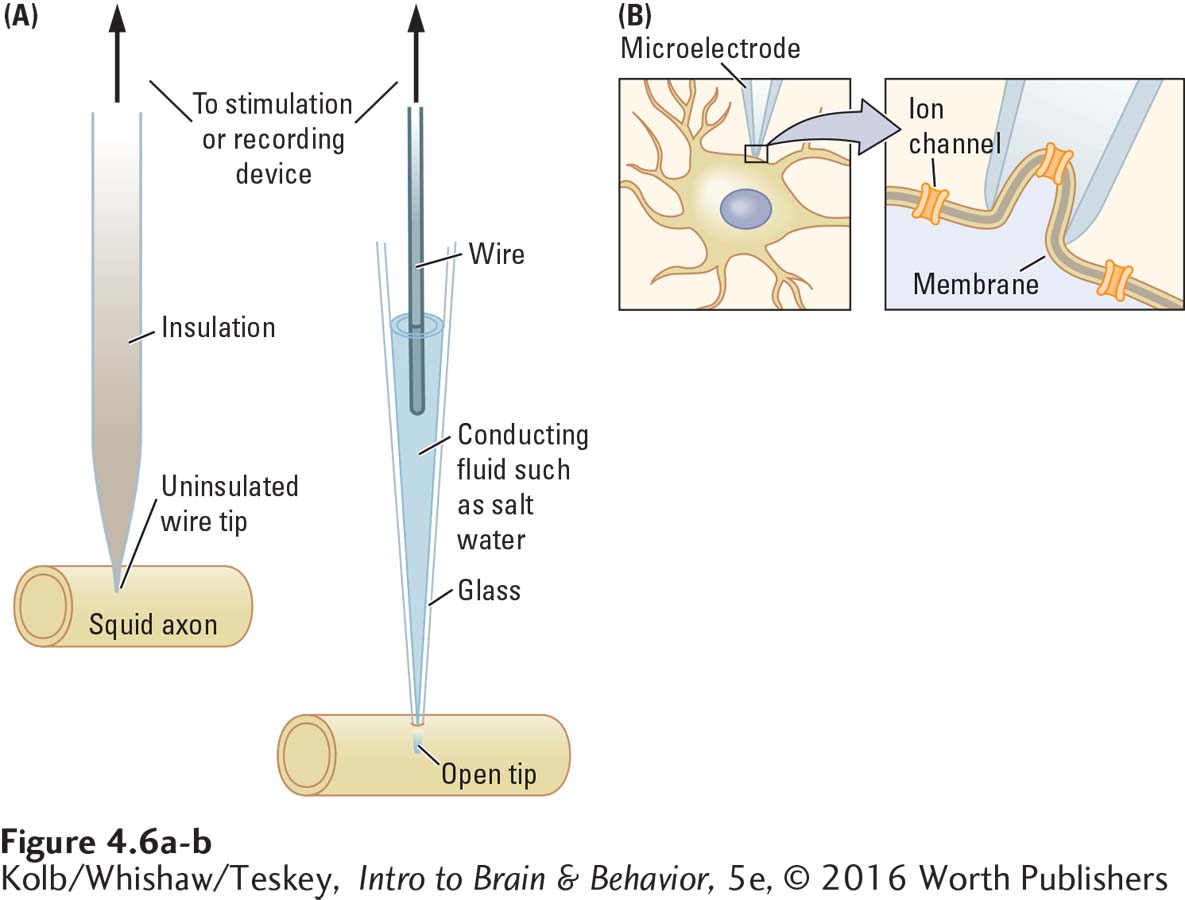
The final device needed to measure a neuron’s electrical activity is an electrode small enough to place on or in an axon—
Microelectrodes can also be made from a thin glass tube tapered to a very fine tip (Figure 4-6A, right image). The tip of a hollow glass microelectrode can be as small as 1 µm. When the glass tube is filled with salty water, a conducting medium (through which an electrical current can travel), it acts as an electrode. A wire in the salt solution connects the electrode to either a stimulating or a recording device.
Microelectrodes can record from axons in many ways. The tip of a microelectrode placed on an axon provides an extracellular measure of the electrical current from a tiny part of the axon. The tip of one electrode can be placed on the surface of the axon and the tip of a second electrode can be inserted into the axon. This technique measures voltage across the cell membrane.
A still more refined use of a glass microelectrode is to place its tip on the neuron’s membrane and apply a little suction until the tip is sealed to a patch of the membrane, as shown in Figure 4-6B. This technique, analogous to placing the end of a soda straw against a piece of plastic wrapping and sucking, allows a recording to be made from only the small patch of membrane sealed to the microelectrode tip.
Using the giant axon of the squid, an oscilloscope, and microelectrodes, Hodgkin and Huxley recorded the electrical voltage on an axon’s membrane and explained the nerve impulse as changes in ion concentration across the cell membrane. The basis of electrical activity in nerves is the movement of intracellular and extracellular ions, which carry positive and negative charges across the cell membrane. To understand Hodgkin and Huxley’s results, you first need to understand the principles underlying the movement of ions.
How Ion Movement Produces Electrical Charges
The intracellular fluid within a neuron and the extracellular fluid surrounding it contain various ions, including Na+ (sodium) and K+ (potassium)—positively charged, as the plus signs indicate—
Because molecules move constantly, they spontaneously spread out from a point of concentration. This spreading out is diffusion. Requiring no work, diffusion results from the random motion of molecules as they move and bounce off one another to gradually disperse in a solution. When diffusion is complete, a dynamic equilibrium, with an equal number of molecules everywhere, is the result.
Smoke from a fire gradually diffuses through the air in a room until every bit of air contains the same number of smoke molecules. Dye poured into water diffuses in the same way—
The Basics, pp. 88–89, covers ions. The illustration, Salty Water, shows how water molecules dissolve salt crystals.
Concentration gradient describes the relative abundance of a substance in space or in a solution. As illustrated in Figure 4-7A, when you drop a little ink into a beaker of water, the dye starts out concentrated at the site of contact, then diffuses. The ink spreads out from a point of concentration until it is equally distributed and all the water in the beaker is the same color. A similar process takes place when a salt is put into water. The salt is initially concentrated where it enters the water, but it diffuses from that location until its ions are in equilibrium.
Because ions carry an electrical charge and because like charges repel one another, ion movement can be described either by a concentration gradient, the difference in the number of ions between two regions, or by a voltage gradient, the difference in charge between two regions. Ions will move down a voltage gradient from an area of higher charge to an area of lower charge, just as they move down a concentration gradient from an area of higher concentration to an area of lower concentration.
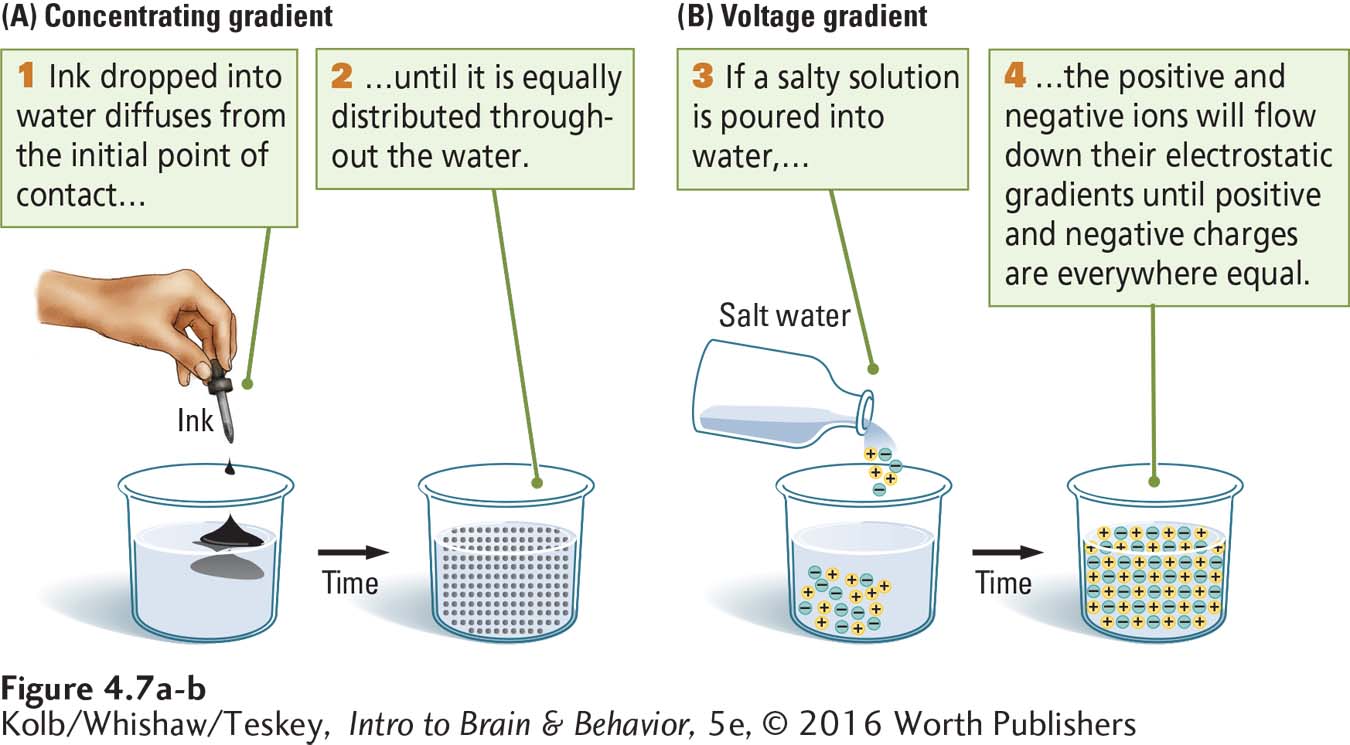
Figure 4-7B illustrates this process. When salt is dissolved in water, its diffusion can be described either as movement down a concentration gradient (for sodium and chloride ions) or movement down a voltage gradient (for the positive and negative charges). In a container that allows unimpeded movement of ions, the positive and negative charges eventually balance.
The cell membrane is an insulator impermeable to salty solutions: salt ions, surrounded by water molecules, will not pass through the membrane’s hydrophobic tails (review Figure 3-11).
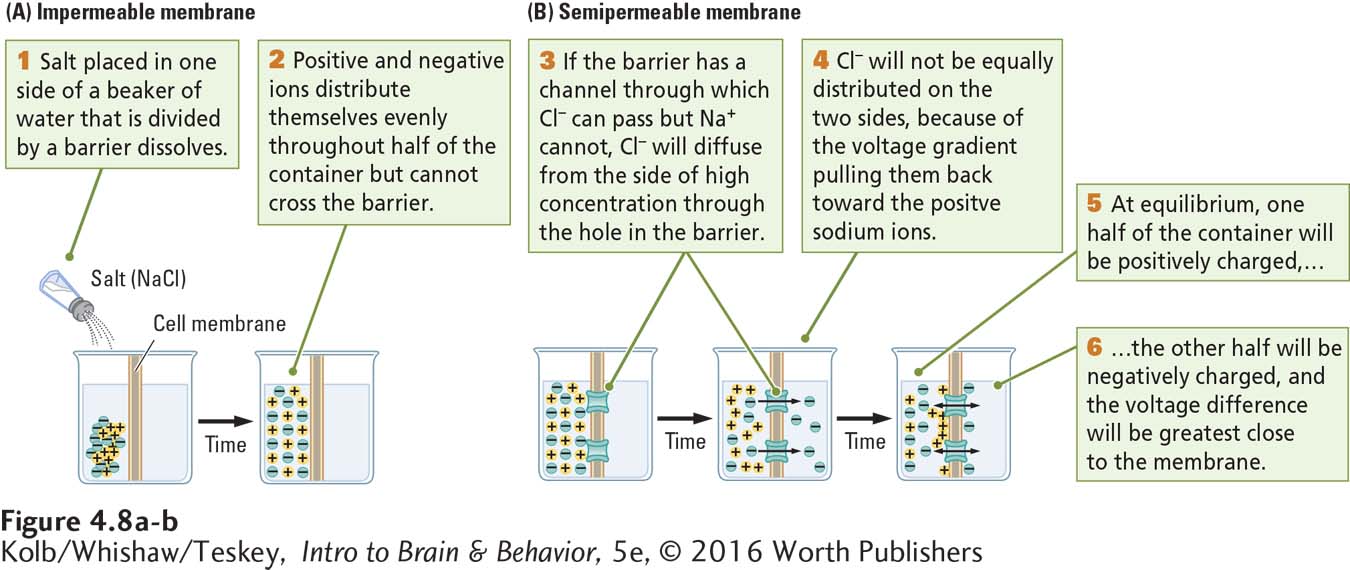
A thought experiment will illustrate how a cell membrane influences ion movement. Figure 4-8A shows a container of water divided in half by a solid, impermeable membrane. If we place a few grains of table salt (NaCl) in the left half of the container, the salt dissolves. The ions diffuse down their concentration and voltage gradients until the water in the left compartment is in equilibrium.
In the left side of the container, there is no longer a gradient for either sodium or chloride ions because the water everywhere is equally salty. There are no gradients for these ions on the other side of the container either, because the solid membrane prevents the ions from entering that side. But there are concentration and voltage gradients for both sodium and chloride ions across the membrane—
Dissolved sodium ions are smaller than chloride ions but more likely to stick to water molecules and so are bulkier and will not pass through a small chloride channel.
Transmembrane protein molecules embedded in a cell membrane form channels, some with gates, and pumps that allow certain kinds of ions to pass through the membrane. Returning to our thought experiment, we place a few chloride channels in the membrane that divides the container of water, making the membrane semipermeable, as illustrated at the left in Figure 4-8B. Chloride ions will now diffuse across the membrane and move down their concentration gradient on the side of the container that previously had no chloride ions, shown in the middle of Figure 4-8B. The sodium ions, in contrast, cannot cross through the chloride channels or the cell membrane.
If the only factor affecting the movement of chloride ions were the chloride concentration gradient, the efflux (outflow) of chloride from the salty to the freshwater side of the container would continue until chloride ions were in equilibrium on both sides. But this is not what happens. Because opposite charges attract, the chloride ions, which carry a negative charge, are attracted to the positively charged sodium ions they left behind. Because they are pulled back toward the sodium ions, the chloride ions cannot diffuse completely. Consequently, the concentration of chloride ions remains higher in the left side of the container than in the right, as illustrated at the right in Figure 4-8B.
In other words, the efflux of chloride ions down the chloride concentration gradient is counteracted by the influx (inflow) of chloride ions down the chloride voltage gradient. At some point, equilibrium is reached: the chloride concentration gradient on the right side of the beaker is balanced by the chloride voltage gradient on the left. In brief:
concentration gradient = voltage gradient
At this equilibrium, the differential concentration of the chloride ions on the two sides of the membrane produces a difference in charge—
4-1 REVIEW
Searching for Electrical Activity in the Nervous System
Before you continue, check your understanding.
Question 1
Although he was incorrect, __________ was the first to seriously attempt to explain how information travels through the nervous system.
Question 2
Experimental results obtained over hundreds of years from electrical __________ and more recently from electrical __________ implicated electrical activity in the nervous system’s flow of information.
Question 3
By the mid-
Question 4
The electrical activity of neuronal axons entails the diffusion of ions. Ions may move down a(n) __________ and down a(n) __________.
Question 5
In what three ways does the semipermeable cell membrane affect the movement of ions in the nervous system?
Answers appear in the Self Test section of the book.