Mitotic CDKs Promote Mitotic Spindle Formation
A key function of mitotic CDKs is to induce the formation of the mitotic spindle, also known as the mitotic apparatus. As we saw in Chapter 18, the mitotic spindle is made of microtubules that attach to chromosomes via specialized protein structures associated with the chromosomes, known as kinetochores. In most organisms, the mitotic spindle is organized by centrosomes, sometimes called spindle pole bodies. The centrosomes contain a specialized tubulin, γ tubulin, which, together with associated proteins, nucleates microtubules. Notable exceptions to these centrosome-
The function of the mitotic spindle is to segregate chromosomes so that the sister chromatids separate from each other and are moved to opposite poles of the cell (see Figure 18-38). To achieve this, the mitotic spindle must attach to the chromosomes so that one kinetochore of each sister chromatid pair attaches to microtubules emanating from opposite spindle poles. Sister chromatids are then said to be bi-
During G1, cells contain a single centrosome, which functions as the major microtubule nucleating center of the cell. Mitotic spindle formation begins at the G1–S phase transition with the duplication of the centrosome. The mechanism whereby this duplication occurs is poorly understood, but at the heart of this process is the duplication of the pair of centrioles, short microtubules arranged orthogonally to each other. As discussed in Chapter 18, G1 cells contain a single pair of centrioles. Concomitant with entry into S phase and triggered by the G1/S phase CDKs, the two centrioles split apart, and each centriole begins to grow a daughter centriole (see Figure 18-36). The new centrioles grow and mature during S phase, each centriole pair begins to assemble centrosomal material, and by G2 the two centrosomes have formed. Several additional protein kinases have been identified that control centrosome duplication. Chief among them is a member of the conserved Polo kinase family, Plk4. How G1/S phase CDKs and Plk4 promote centrosome duplication is not yet understood, but is thought to involve the phosphorylation of multiple centrosome components, which facilitates their duplication and growth. As we will see, the Polo kinases not only play a key role in centrosome duplication, but also participate in essentially all aspects of mitosis.
Page 898
The key initiating step of mitotic spindle formation is the severing of the ties that link the duplicated centrosomes. This centrosome disjunction occurs in G2 and is triggered by mitotic CDKs (see Figure 18-36). As soon as this separation occurs, microtubules extend from both centrosomes, and the two centrosomes move away from each other, pulled by the motor protein dynein. The specifics of microtubule array formation and mitotic spindle assembly were discussed in Chapter 18. Here we briefly consider how chromosomes attach to the mitotic spindle and how mistakes in the process are corrected.
For chromosomes to be accurately segregated during mitosis, the sister chromatid pair must be stably bi-
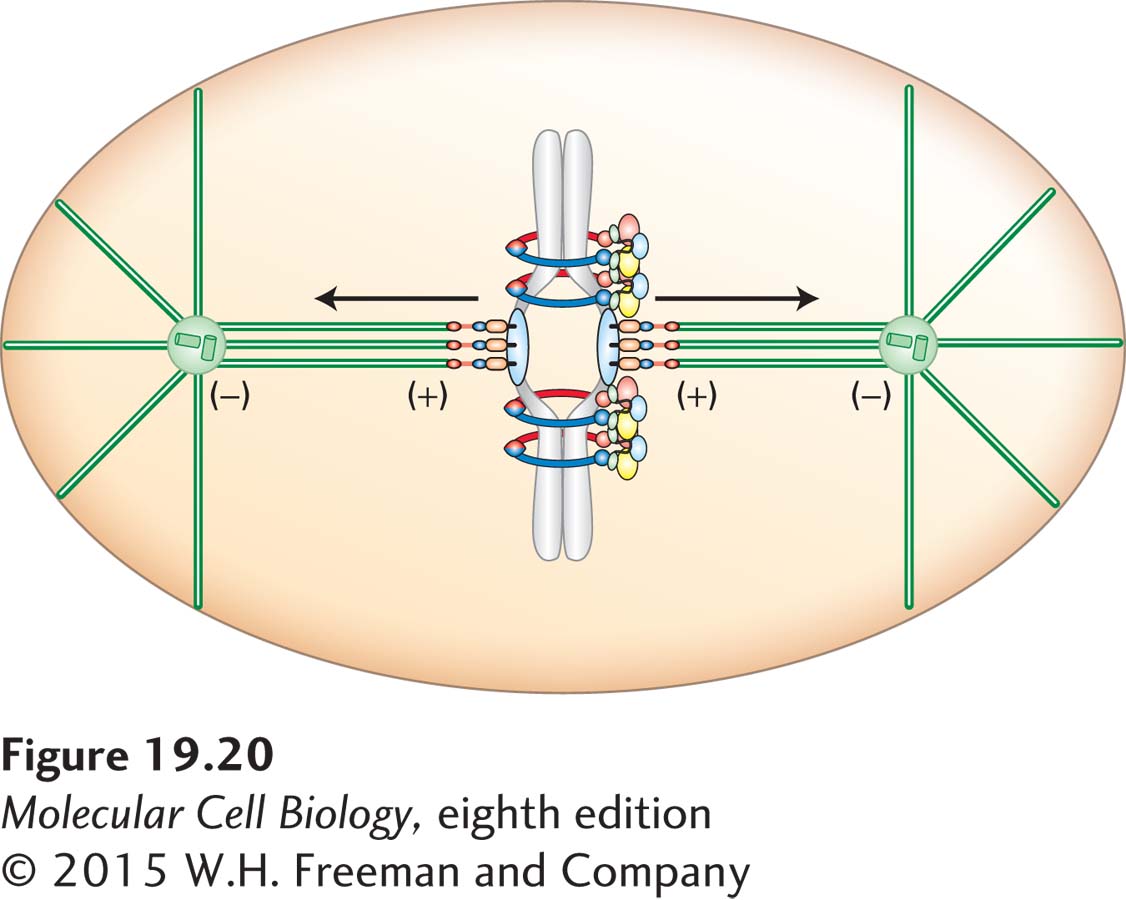
The ultimate goal of chromosome attachment to the mitotic spindle is that each and every chromosome be attached to the mitotic spindle in a bi-
Page 899
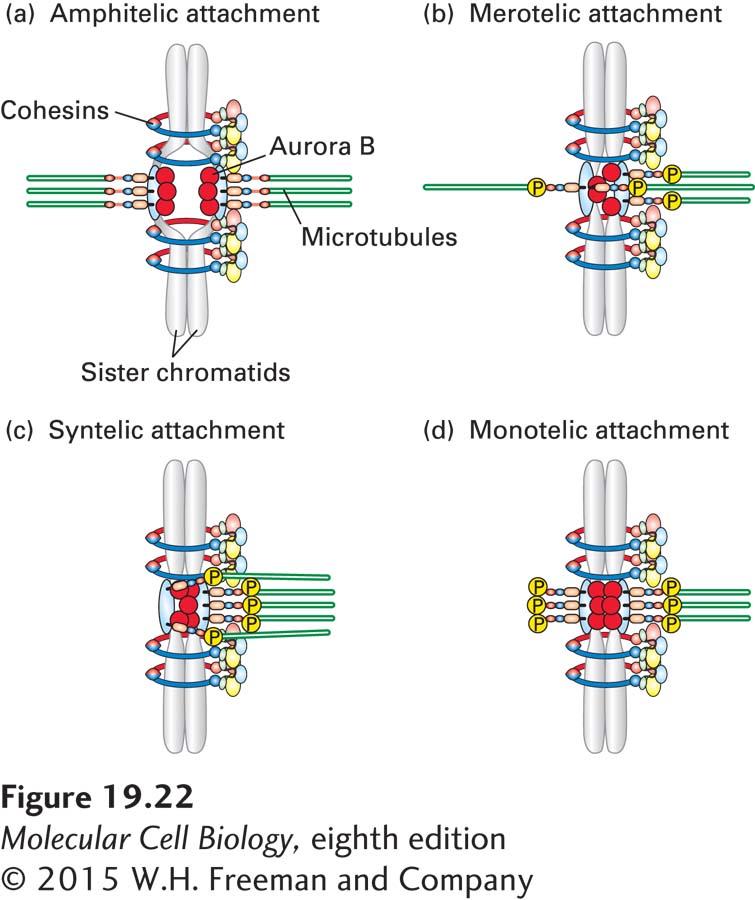
The sensing mechanism used by cells to detect incorrect attachments is based on tension. When sister chromatids are correctly attached to microtubules, their kinetochores are under tension (see Figure 19-22a). Microtubules attached to the kinetochores pull at them, and the cohesin molecules that hold the sister chromatids together withstand these forces, creating tension at kinetochores. Merotelic, syntelic, or monotelic attachment leads to insufficient tension at kinetochores, allowing the cell to distinguish these faulty forms of attachment from the correct amphitelic one.
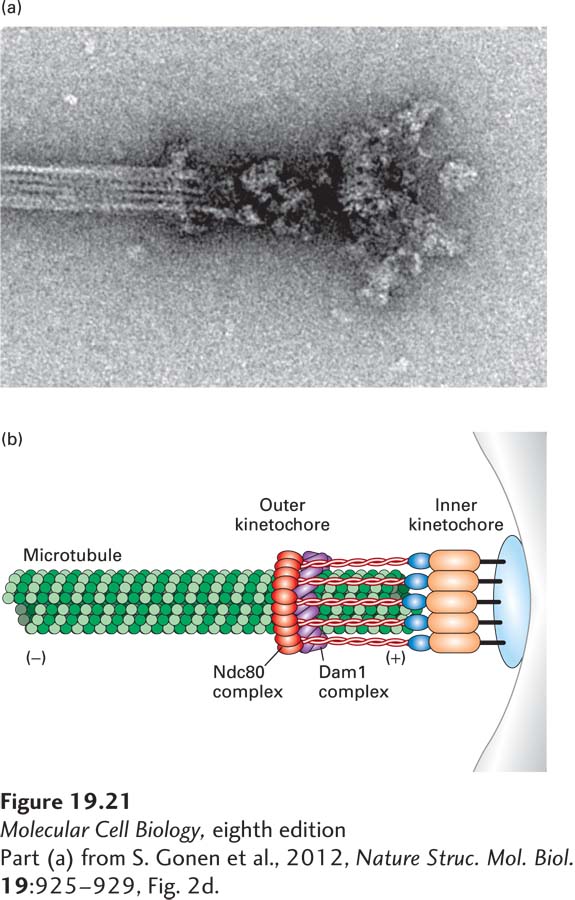
How does the cell sense whether or not kinetochores are under tension? The protein kinase Aurora B and its associated regulatory factors, together known as the chromosomal passenger complex (CPC), sense kinetochores that are not under tension and sever these microtubule attachments, giving cells a second chance to get the attachment right. The molecular basis for this sensing mechanism is partly understood. Recall that outer kinetochore components, especially the Ndc80 complex, bind microtubules (see Figure 18-41). Aurora B phosphorylates Ndc80. When phosphorylated, the protein loses its microtubule-
Microtubules continuously pull on chromosomes. Once all chromosomes have attached to microtubules in an amphitelic manner, the only thing that prevents chromosomes from segregating to the poles is the cohesins that hold them back in the middle of the spindle (see Figure 19-22a). As we will see in Section 19.6, it is the severing of these cohesins that initiates anaphase chromosome segregation.