Phospholipids Associate Noncovalently to Form the Basic Bilayer Structure of Biomembranes
Biomembranes are large, flexible sheets with a two-
To understand the structure a phospholipid molecule, we have to understand each of its component parts and how it is assembled. As we will see shortly, a phospholipid molecule consists of two long-
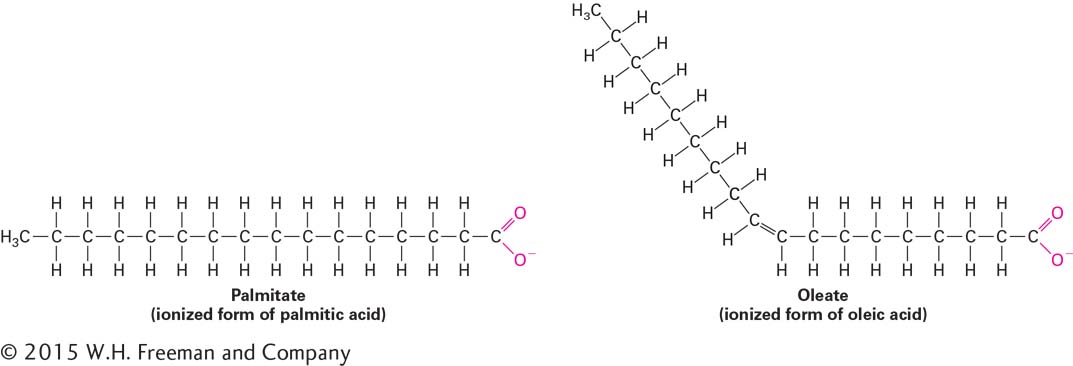
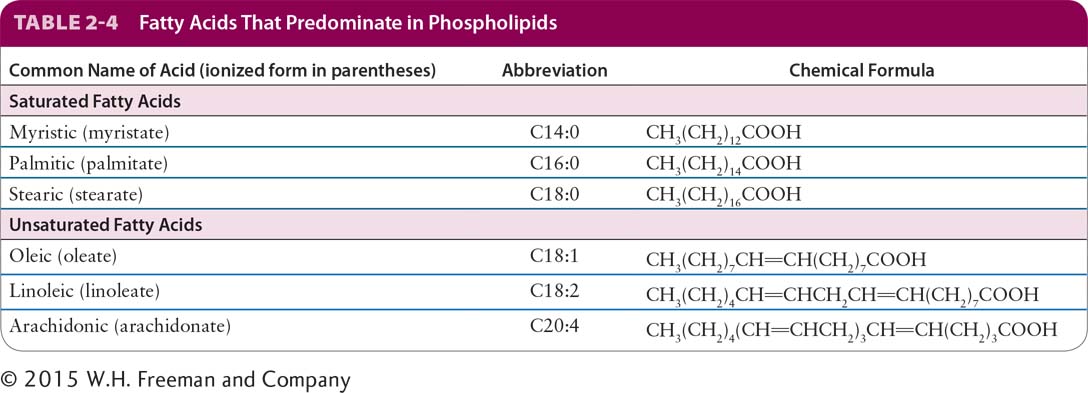
Fatty acids consist of a hydrocarbon chain attached to a carboxyl group (–COOH). Like glucose, fatty acids are an important energy source for many cells (see Chapter 12). They differ in length, although the predominant fatty acids in cells have an even number of carbon atoms, usually 14, 16, 18, or 20. The major fatty acids in phospholipids are listed in Table 2-4. Fatty acids are often designated by the abbreviation Cx:y, where x is the number of carbons in the chain and y is the number of double bonds. Fatty acids containing 12 or more carbon atoms are nearly insoluble in aqueous solutions because of their long hydrophobic hydrocarbon chains.
Fatty acids in which all the carbon-
Page 49
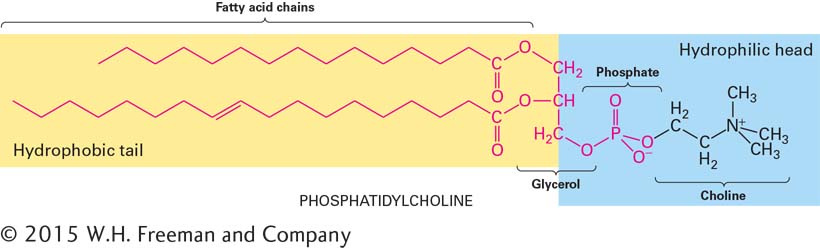
In phospholipids, fatty acids are covalently attached to another molecule by esterification. In the combined molecule formed by this reaction, the part derived from the fatty acid is called an acyl group, or fatty acyl group. This structure is illustrated by the most common forms of phospholipids: phosphoglycerides, which contain two acyl groups attached to two of the three hydroxyl groups of glycerol (see Figure 2-20).
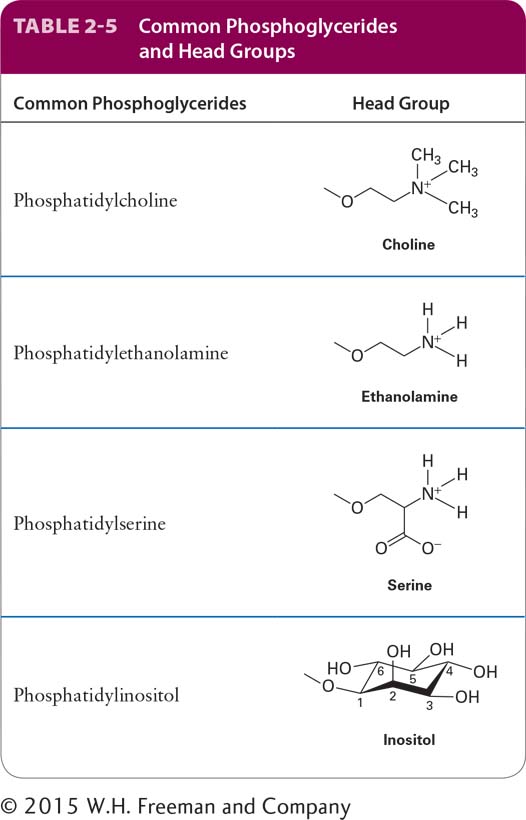
In phosphoglycerides, one hydroxyl group of the glycerol is esterified to phosphate while the other two are normally esterified to fatty acids. The simplest phospholipid, phosphatidic acid, contains only these components. Phospholipids such as phosphatidic acids are not only membrane building blocks but also important signaling molecules. Lysophosphatidic acid, in which the acyl chain at the 2 position (attached to the hydroxyl group on the central carbon of the glycerol) has been removed, is relatively water soluble and can be a potent inducer of cell division (called a mitogen). In most phospholipids found in membranes, the phosphate group is also esterified to a hydroxyl group on another hydrophilic compound. In phosphatidylcholine, for example, choline is attached to the phosphate (see Figure 2-20). The negatively charged phosphate, as well as the charged or polar groups esterified to it, can interact strongly with water. The phosphate and its associated esterified group constitute the “head” group of a phospholipid, which is hydrophilic, whereas the fatty acyl chains, the “tails,” are hydrophobic. Other common phosphoglycerides and associated head groups are shown in Table 2-5. Molecules such as phospholipids that have both hydrophobic and hydrophilic regions are called amphipathic. In Chapter 7, we will see how the amphipathic properties of phospholipids allow their assembly into sheet-
Fatty acyl groups also can be covalently linked in other fatty molecules, including triacylglycerols, or triglycerides, which contain three acyl groups esterified to glycerol:
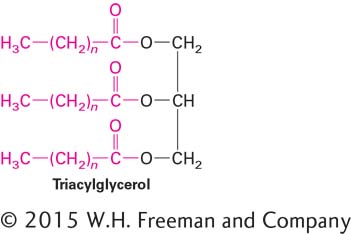
Page 50
They also can be covalently attached to the very hydrophobic molecule cholesterol, an alcohol, to form cholesteryl esters:
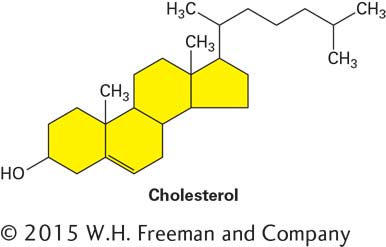
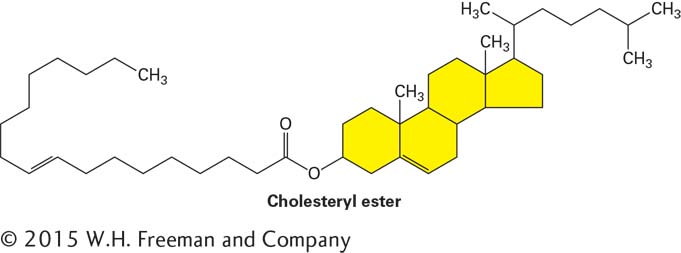
Triglycerides and cholesteryl esters are extremely water-
![]() We saw above that the fatty acids, which are key components of both phospholipids and triglycerides, can be either saturated or unsaturated. An important consequence of the carbon-
We saw above that the fatty acids, which are key components of both phospholipids and triglycerides, can be either saturated or unsaturated. An important consequence of the carbon-
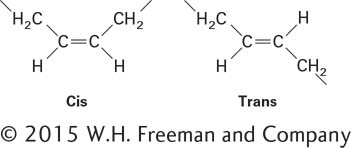
A cis double bond introduces a rigid kink in the otherwise flexible straight acyl chain of a saturated fatty acid (Figure 2-21). In general, the unsaturated fatty acids in biological systems contain only cis double bonds. Saturated fatty acids without the kink can pack together tightly and so have higher melting points than unsaturated fatty acids. The main fatty molecules in butter are triglycerides with saturated fatty acyl chains, which is why butter is usually solid at room temperature. Unsaturated fatty acids or fatty acyl chains with the cis double bond kink cannot pack as closely together as saturated fatty acyl chains. Thus vegetable oils, composed of triglycerides with unsaturated fatty acyl groups, usually are liquid at room temperature. Vegetable and similar oils may be partially hydrogenated to convert some of their unsaturated fatty acyl chains to saturated fatty acyl chains. As a consequence, the hydrogenated vegetable oil can be molded into solid sticks of margarine. A by-
Page 51