Hydrolysis of ATP Releases Substantial Free Energy and Drives Many Cellular Processes
In almost all organisms, the nucleoside triphosphate adenosine triphosphate, or ATP (Figure 2-31), is the most important molecule for capturing, transiently storing, and subsequently transferring energy to perform work (e.g., biosynthesis, mechanical motion). Commonly referred to as a cell’s energy “currency,” ATP is a type of usable potential energy that cells can “spend” in order to power their activities. The storied history of ATP begins with its discovery in 1929, apparently simultaneously by Kurt Lohmann, who was working with the great biochemist Otto Meyerhof in Germany and who published first, and by Cyrus Fiske and Yellapragada SubbaRow in the United States. Muscle contractions were shown to depend on ATP in the 1930s. The proposal that ATP is the main intermediary for the transfer of energy in cells is credited to Fritz Lipmann around 1941. Many Nobel Prizes have been awarded for the study of ATP and its role in cellular energy metabolism, and its importance in understanding molecular cell biology cannot be overstated.
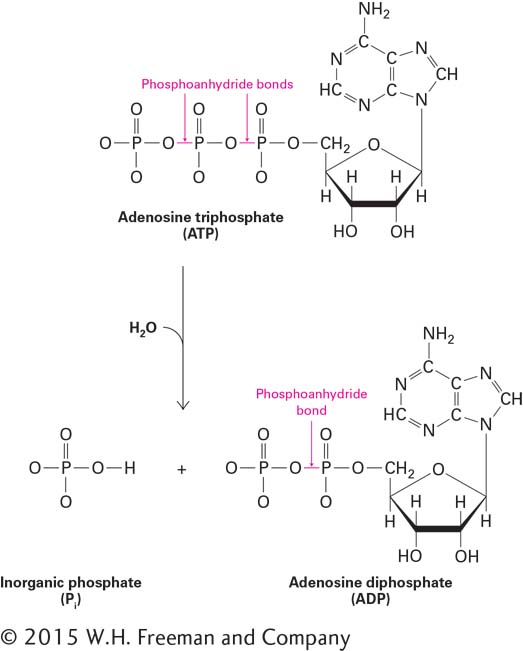
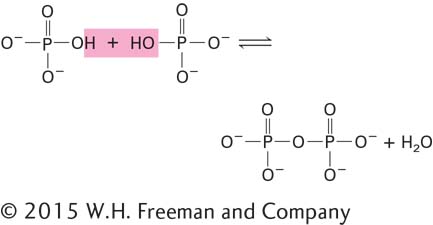
The useful energy in an ATP molecule is contained in phosphoanhydride bonds, which are covalent bonds formed from the condensation of two molecules of phosphate by the loss of water:
Page 62
As shown in Figure 2-31, an ATP molecule has two key phosphoanhydride (also called phosphodiester) bonds. Forming these bonds (represented here by the symbol ∼) in ATP requires an input of energy. When these bonds are hydrolyzed, or broken by the addition of water, that energy is released. Hydrolysis of a phosphoanhydride bond in each of the following reactions has a highly negative ΔG°′ of about –7.3 kcal/mol:
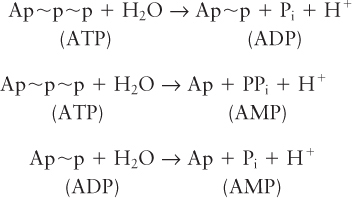
Pi stands for inorganic phosphate (PO43–
A phosphoanhydride bond or other “high-
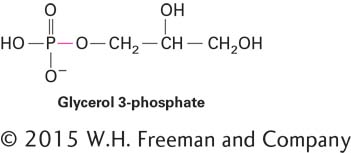
A principal reason for this difference is that ATP and its hydrolysis products, ADP and Pi, are charged at neutral pH. During synthesis of ATP, a large amount of energy must be used to force the negative charges in ADP and Pi together. Conversely, this energy is released when ATP is hydrolyzed to ADP and Pi. In comparison, formation of the phosphoester bond between an uncharged hydroxyl in glycerol and Pi requires less energy, and less energy is released when this bond is hydrolyzed.
Cells have evolved protein-
B + Ap ~p~p → B~p + Ap~p
B~p + C → D + Pi
The overall reaction
B + C + ATP ⇌ D + ADP + Pi
is energetically favorable (ΔG < 0). Similarly, hydrolysis of GTP to GDP can provide energy to perform work, including the synthesis of ATP (see Chapter 12), but most often GTP hydrolysis is used to control cellular systems (e.g., protein synthesis, hormonal signaling) rather than as a source of energy.
An alternative mechanism of energy coupling is to use the energy released by ATP hydrolysis to change the conformation of a molecule to an “energy-
As with many biosynthetic reactions, transport of molecules into or out of the cell often has a positive ΔG and thus requires an input of energy to proceed. Such simple transport reactions do not directly involve the making or breaking of covalent bonds; thus their ΔG°′ is 0. In the case of a substance moving into a cell, Equation 2-
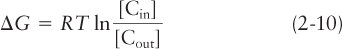
where [Cin] is the initial concentration of the substance inside the cell and [Cout] is its concentration outside the cell. We can see from Equation 2-