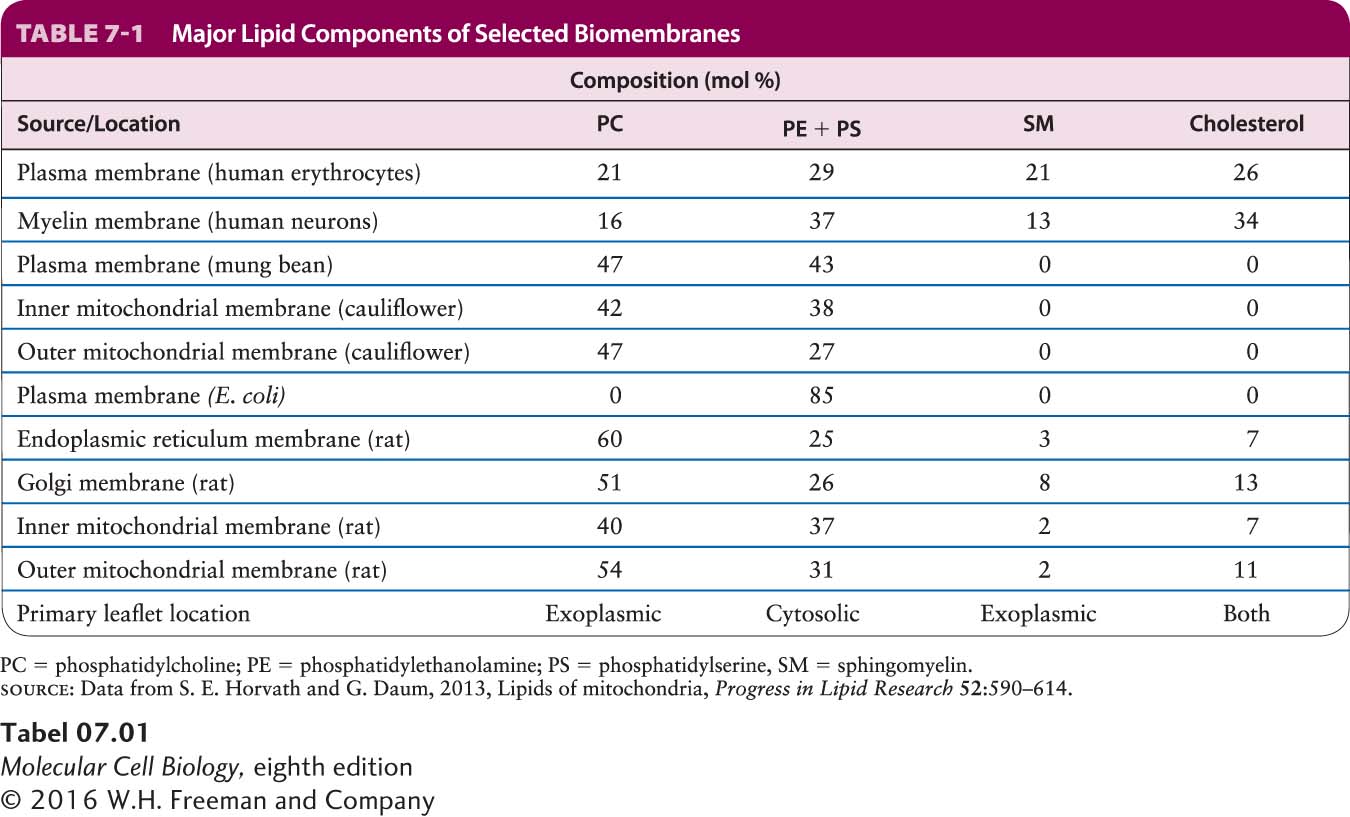
Lipid Composition Influences the Physical Properties of Membranes
A typical cell contains many different types of membranes, each with unique properties derived from its particular mix of lipids and proteins. The data in Table 7-1 illustrate the variation in lipid composition in different biomembranes. Several phenomena contribute to these differences. For instance, the relative abundances of phosphoglycerides and sphingolipids differ between membranes in the endoplasmic reticulum (ER), where phospholipids are synthesized, and the Golgi complex, where sphingolipids are synthesized. The proportion of sphingomyelin as a percentage of total membrane lipid phosphorus is about six times as high in Golgi membranes as it is in ER membranes. In other cases, the movement of membranes from one cellular compartment to another can selectively enrich certain membranes in lipids such as cholesterol. In responding to differing environments throughout an organism, different types of cells generate membranes with differing lipid compositions. In the cells that line the intestinal tract, for example, the membranes that face the harsh environment in which dietary nutrients are digested have a sphingolipid-
The degree of bilayer fluidity depends on lipid composition, the structure of the phospholipid hydrophobic tails, and temperature. As already noted, van der Waals interactions and the hydrophobic effect cause the nonpolar tails of phospholipids to aggregate. Long, saturated fatty acyl chains have the greatest tendency to aggregate, packing tightly together into a gel-
Page 280
Cholesterol is important in maintaining the appropriate fluidity of natural membranes, a property that appears to be essential for normal cell growth and reproduction. Cholesterol restricts the random movement of phospholipid head groups at the outer surfaces of the leaflets, but its effect on the movement of long phospholipid tails depends on its concentration. At the cholesterol concentrations normally present in the plasma membrane, the interaction of the steroid ring with the long hydrophobic tails of phospholipids tends to immobilize those lipids and thus decreases biomembrane fluidity. It is this property that can help organize the plasma membrane into discrete subdomains of unique lipid and protein composition. At lower cholesterol concentrations, however, the steroid ring separates and disperses phospholipid tails, causing the inner regions of the membrane to become slightly more fluid.
The lipid composition of a bilayer also influences its thickness, which in turn may influence the distribution of other membrane components, such as proteins, in a particular membrane. It has been argued that relatively short transmembrane segments of certain Golgi-
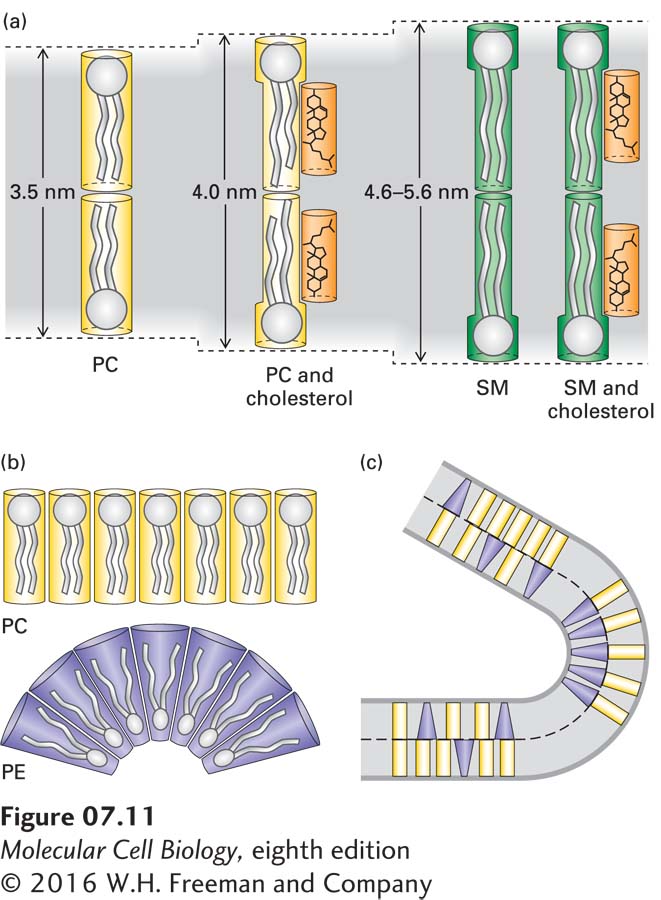
Another property dependent on the lipid composition of a bilayer is its curvature, which depends on the relative sizes of the polar head groups and nonpolar tails of its constituent phospholipids. Lipids with long tails and large head groups are cylindrical in shape; those with small head groups are cone-
Page 281