The carbon cycle is closely tied to the movement of energy
Because all organisms are composed of carbon, the movement of carbon in ecosystems largely follows the same paths as the movement of energy. In this section, we consider the many pools and processes that are involved in the carbon cycle. We will then examine how human activities have altered the carbon cycle.
The Carbon Cycle
To understand how the carbon cycle works, we need to consider six types of transformations: photosynthesis, respiration, sedimentation and burial, exchange, extraction, and combustion. The carbon cycle is illustrated in Figure 21.3.
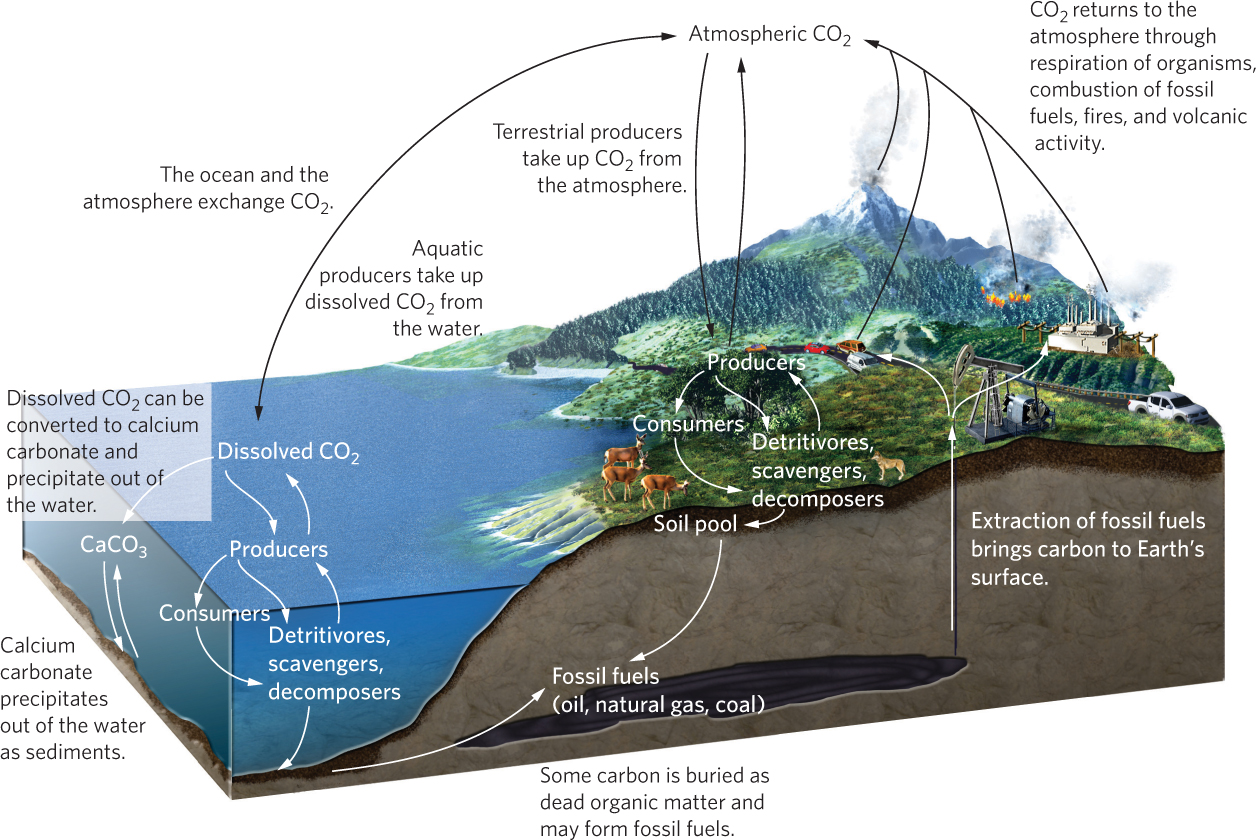
We begin our examination with the processes of photosynthesis and respiration. As we have discussed in previous chapters, producers use photosynthesis in terrestrial and aquatic ecosystems to take CO2 from the air and water and to convert it to carbohydrates. These carbohydrates are used to make other compounds including proteins and fats. The carbon that is locked up in producers can then be transferred to consumers, scavengers, detritivores, and decomposers. All of these trophic groups experience respiration, which releases CO2 back into the air or water.
495
In some habitats, such as the waterlogged sediments of swamps or marshes, oxygen is not available to serve as a terminal electron acceptor for respiration. Under such anaerobic conditions, some species of archaea use carbon compounds to accept the electrons. For example, some archaea use methanol (CH3OH) during respiration to produce CO2 in the following reaction:
4 CH3OH → CO2 + 2 H2O + 3 CH4
As you can see from this reaction, the products are carbon dioxide, water, and methane. The methane that is released from swamps during anaerobic respiration is known as swamp gas. The production of methane through the process of anaerobic respiration is a growing concern because methane is a greenhouse gas and, on a per-molecule basis, it is 72 times more effective at absorbing and radiating infrared radiation back to Earth than CO2.
Carbon dioxide is also exchanged between aquatic ecosystems and the atmosphere, as shown in Figure 21.3. The exchange occurs in both directions at a similar magnitude, which means there is little net transfer over time. As we discussed in Chapter 2, when CO2 diffuses from the atmosphere into the ocean, some of it is used by plants and algae for photosynthesis and some is converted to carbonate (CO32–) and bicarbonate ions (HCO3−). The carbonate ions can then combine with calcium in the water to form calcium carbonate (CaCO3). Calcium carbonate has a low solubility in water, so it precipitates out of the water and becomes part of the sediments at the bottom of the ocean. Over millions of years, the calcium carbonate sediments that accumulate in the ocean bottoms, combined with the calcium carbonate skeletons from tiny marine organisms, can develop into massive sources of carbon in the form of rock known as dolomite and limestone. Humans mine dolomite and limestone for use in making concrete and fertilizer as well as for numerous industrial processes.
Carbon can also be buried as organic matter before it fully decomposes. Over millions of years, some of this organic matter is converted to fossil fuels such as oil, gas, and coal. The rate of carbon burial is slow and it is offset by the rate of carbon released into the atmosphere by the weathering of limestone rock and during volcanic eruptions. Because the process of sedimentation and burial can lock up carbon for millions of years, carbon moves through these pools very slowly.
496
The extraction of fossil fuels such as coal, oil, and natural gas represents a recent change to the carbon cycle. Most of human history saw little extraction of carbon that had been buried for millions of years. During the past 2 centuries, however, humans have extracted carbon at an increasing rate to meet growing energy demands. The extraction of fossil fuels moves fossil carbon from underground to Earth’s surface, and the combustion of these fossil fuels alters the carbon cycle significantly.
Combustion of carbon sources produces CO2 that goes into the atmosphere. Some combustion is natural, such as fires occurring in forests and grasslands. Other combustion is caused by humans, such as burning land to prepare it for agriculture and burning fossil fuels to generate electricity and heat or to power machinery. Like respiration and decomposition, combustion converts organic compounds to CO2.
Human Impacts on the Carbon Cycle
Now that we understand the pools and processes in the carbon cycle, we can examine how human activities have altered it. In Chapter 4 we discussed the recent rise in atmospheric CO2 seen in measurements made atop Mauna Loa on the island of Hawaii. These measurements have documented an increase in CO2 from 316 ppm in 1958 to 394 ppm in 2012—a 25 percent increase in just 54 years.
Although these measurements at Mauna Loa began only in 1958, human activities have affected CO2 concentrations in the atmosphere for much longer. To measure the concentrations of CO2 that were in the atmosphere hundreds of thousands of years ago, researchers have traveled to some of the coldest places on Earth. In locations such as Greenland and Antarctica, snowfall slowly compresses into ice that has tiny bubbles of air trapped inside. Because these bubbles represent tiny samples of the air from thousands of years ago, they can tell us about the climate conditions in the distant past. Each year, ice is formed by adding a new layer; the surface layers contain the youngest ice and the deepest layers contain the oldest ice. To sample the air that is trapped in the ice, researchers drill far down into the ice and extract long cylinders of ice known as ice cores (Figure 21.4).

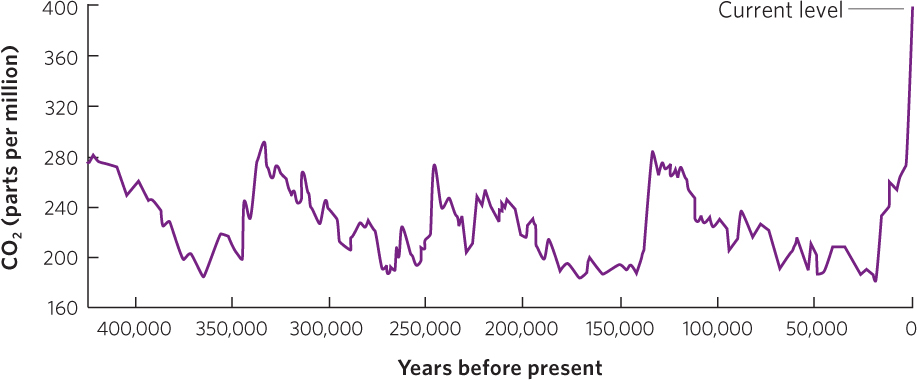
Ice cores represent ice that has been formed as far back as 500,000 years ago. After determining the age of different ice core layers, researchers melt each layer, which allows the release of trapped air bubbles so the concentration of CO2 can be measured. You can view some of the data from these ice cores in Figure 21.5. As you can see, CO2 concentrations in the atmosphere during the past 400,000 years have varied a great deal, from about 180 to 300 ppm. However, since 1800, as humans increasingly burned fossil fuels, CO2 concentrations have increased exponentially to the current value of 394 ppm. This means that the current concentration of CO2 in our atmosphere is 31 percent higher than the highest concentrations that existed during the past 400,000 years.
The rise in atmospheric CO2 is of great importance to humans because CO2 is a greenhouse gas that absorbs infrared radiation and radiates some of it back to Earth. Having CO2 in the atmosphere helps keep our planet warm, but an excessive amount of CO2 and other greenhouse gases in the atmosphere could cause our planet to become much warmer than it has been in a very long time. We know that the mean temperature on Earth is now 0.8°C warmer than it was when the first temperature measurements were taken in the 1880s. While a mean increase of 0.8°C may not seem like much, we find some dramatic changes in specific locations. Some regions, such as parts of Antarctica, have experienced cooler temperatures while other regions, such as the high latitudes of Alaska, Canada, and Russia, are now 4°C warmer than they were a century ago.
497
These high-latitude regions contain large deposits of frozen peat, which is a mixture of dead sphagnum moss and other plants. Peat thaws and decomposes more easily with warmer temperatures. Because peat decomposes under anaerobic conditions, the decomposition produces methane, which is a greenhouse gas. This means that the rise in temperatures due to increased atmospheric CO2 causes the release of additional greenhouse gases from the decomposing peat that exacerbate the problem. Temperature increases can have numerous effects around the world, such as reducing the size of the polar ice sheets (see Chapter 5), altering the length of plant growing seasons, and changing the timing of plant and animal life histories. We will have much more to say about global warming in our discussion of global biodiversity conservation in Chapter 23.