Acidification threatens life in the world’s oceans.
From the beginning of the Industrial Revolution to the present day, we humans have burned enough fossil fuels and clear-cut enough forests to release more than 500 billion metric tons of CO2 into Earth’s atmosphere; thanks to these forces, the atmospheric CO2 level is higher today than at any other point in the past 800,000 years. Even worse than these unprecedented levels is how fast they have risen—too fast, experts say, for many organisms to adapt. Much has been made of what heat-trapping molecules do to terrestrial ecosystems. But, as scientists are now learning, their effects on aquatic environments is just as profound.
Ocean and atmosphere come into direct contact over 75% of Earth’s surface, and they are constantly exchanging gases over that interface; anything emitted into one eventually ends up in the other—including CO2. Winds, waves, and currents quickly mix CO2 into the top few hundred feet of water, and as years pass, currents pull it ever deeper into the ocean. Between the early 1990s and mid-2000s, scientists around the world collected and analyzed nearly 80,000 water samples from a range of ocean environments. By their estimates, some 30% of all the CO2 released by humans in the past two centuries has been absorbed by the world’s oceans. “For terrestrial ecosystems, it’s a good thing,” says Slattery. “Because it means that much less CO2 is lingering in the atmosphere. But for oceans, it could be very bad.”
KEY CONCEPT 29.1
The world’s oceans have absorbed much of the CO2 released from fossil fuel burning, causing the pH of ocean water to fall.
Here’s how: Normal seawater has an average pH of around 8.2, meaning it is slightly basic (alkaline). So far, CO2 emissions have reduced the ocean’s pH by about 0.1. That might not sound like much, but the pH scale is logarithmic, so even small numbers represent large effects. A pH drop of 0.1 corresponds to a 30% increase in ocean water acidity. If present trends continue, by 2100 the ocean’s surface waters will be about 150% more acidic than they were in 1800. In 2003, scientists adopted the phrase “ocean acidification ” to describe this coming catastrophe. INFOGRAPHIC 29.1
acidification
The lowering of the pH of a solution.
The pH scale is a measure of how acidic or basic a solution is. It is a measurement that compares the proportion of hydrogen (H+) or hydroxide (OH−) ions in a solution (ions are charged atoms or molecules). Water (H2O) can dissociate into hydroxide ions (OH−) and hydrogen ions (H+). In pure water, there is always one H+ for every OH−. These solutions are neutral, with a pH of 7.0. Acids have a pH lower than 7.0 and have extra H+ ions in the solution. Bases (alkaline solutions) have a pH higher than 7.0 and have extra OH− ions in the solution.
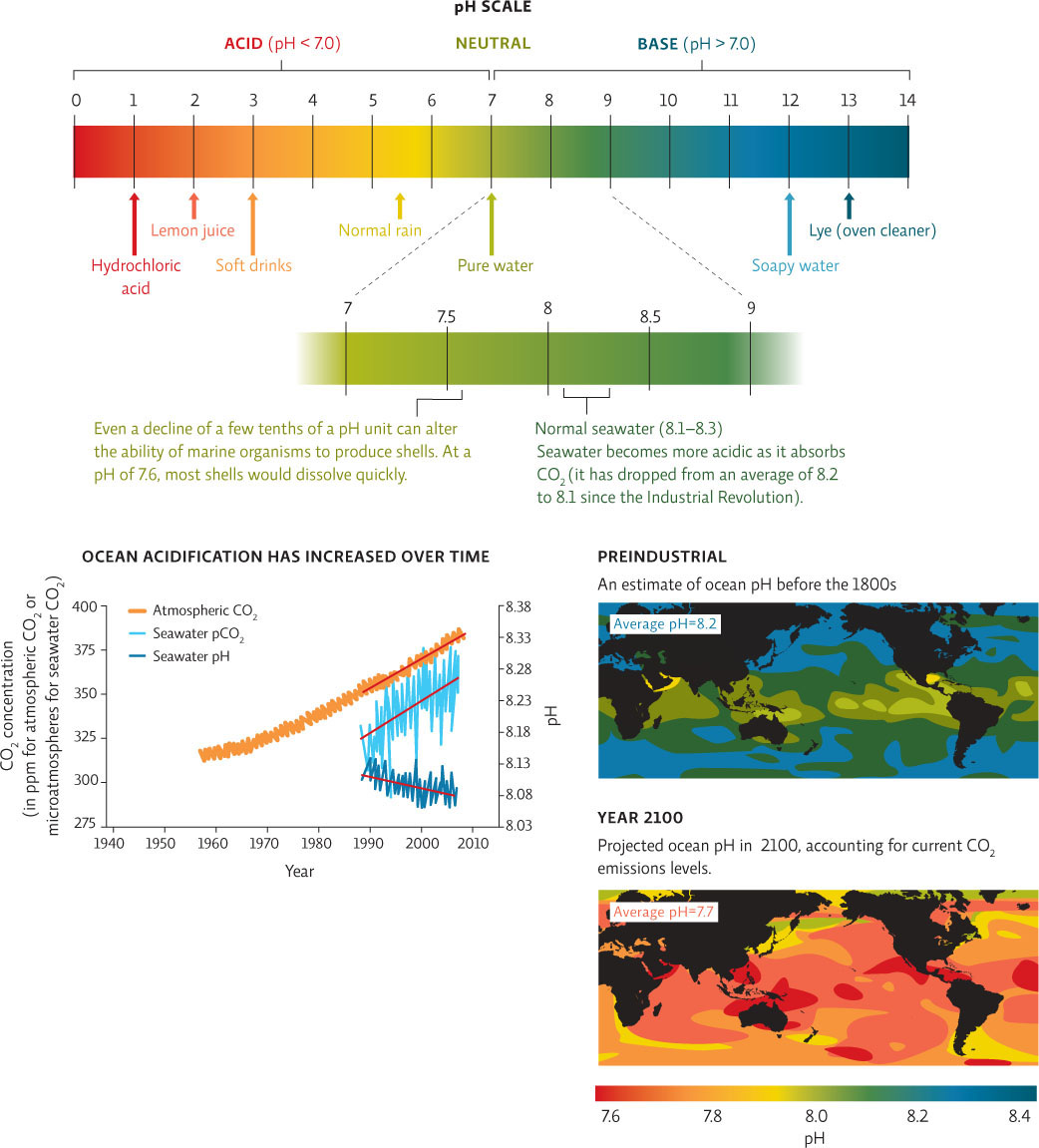
The increase in ocean acidity correlates well with the increases in CO2 dissolved in seawater and atmospheric CO2 concentrations.
Ocean pH will continue to drop significantly if we do not curtail fossil fuel use and our release of extra CO2.

Why are we saying the oceans are becoming acidified when their pH is still alkaline?
The pH is dropping, so relative to the normal pH of about 8.2, this is considered acidification since acids have a lower pH than alkaline solutions.




Scientists expect such a colossal reordering of ocean chemistry to have a huge impact on marine ecosystems. “Ocean acidification is global warming’s equally evil twin,” says Jane Lubchenco, a marine ecologist and the head of the National Oceanic and Atmospheric Administration (NOAA).
One big concern is that as pH shifts, the availability of key nutrients like nitrogen and iron will change, plummeting in some areas, soaring in others, and threatening the stability of virtually all marine ecosystems. In just one example, Michael Berman and his colleagues at the University of California have found that the rate of nitrification (a process that produces nitrate, a form of nitrogen that marine organisms need in order to grow) decreases in tandem with pH; as that happens, smaller species of plankton (which are more tolerant of nitrate declines) gain an advantage over larger ones. Were it pervasive enough, such a change in species composition could alter the food chain and decrease primary production throughout the oceans. (See Chapter 10 for more on food chains and webs.) Indeed, some researchers think this shift may already be occurring. Overall, plankton biomass may have decreased as much as 40% in the 20th century, they say, especially since 1950.
If present trends continue, by 2100 the ocean’s surface waters will be about 150% more acidic than they were in 1800.
The consequences of this particular chain of events are, for now, anybody’s guess. On one hand, there could be a positive feedback effect that amplifies ocean acidification and climate change: Less plankton means that less CO2 is taken in by the organisms, and more is left behind to further acidify the water or reenter the atmosphere. On the other hand, there could be a negative feedback effect on climate change: Less nitrification means less N2O (nitrous oxide—a potent greenhouse gas) is produced, and thus less is released into the atmosphere.
positive feedback
Changes caused by an initial event which accentuate that original event (i.e., changes brought on by warming leading to even more warming).
negative feedback
Reduction or reversal of an effect by its own influence on the process giving rise to it (i.e., changes brought on by warming leading to cooling).
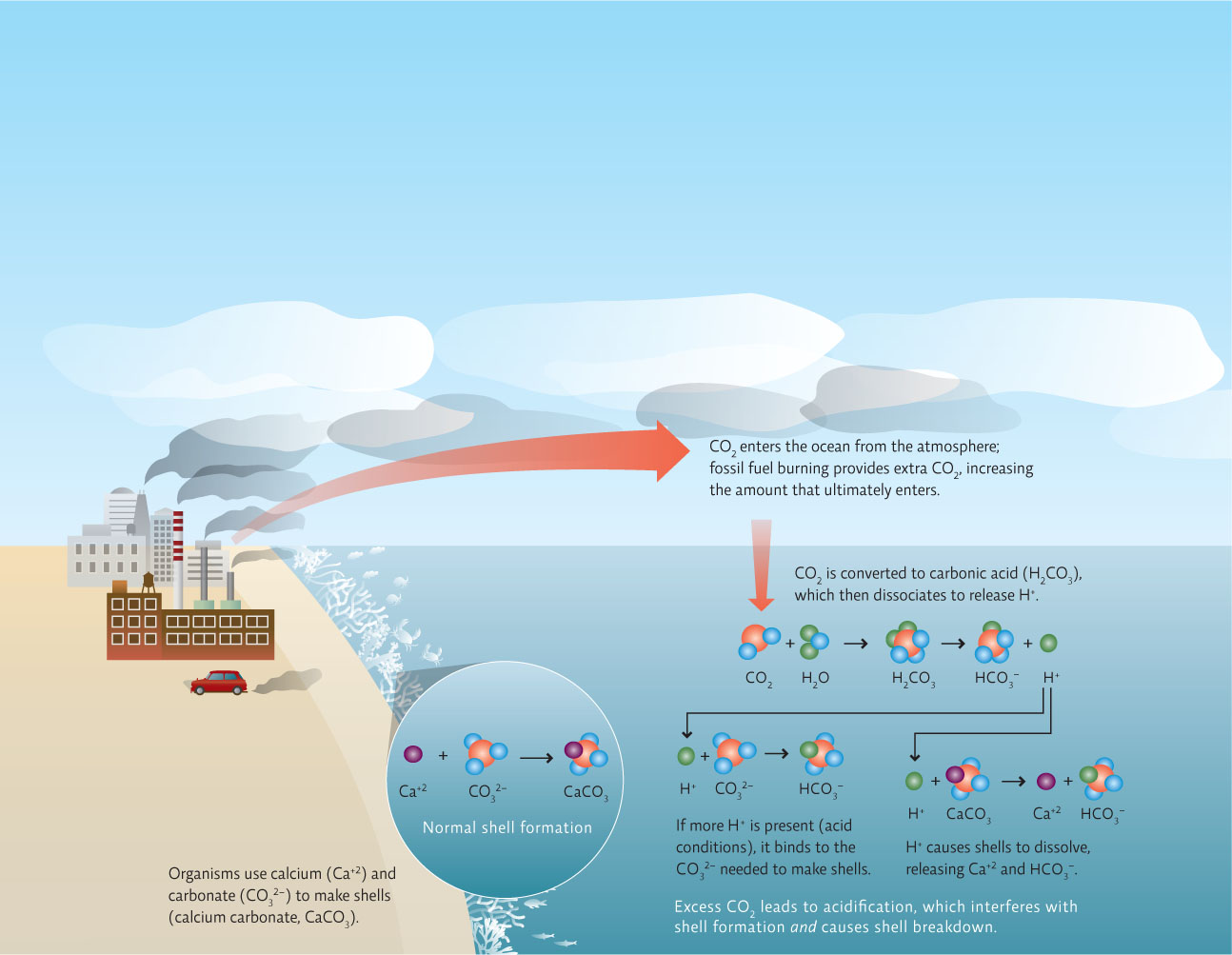
So far, the most well-documented effect of acidification seems to be on marine calcifiers—ocean organisms that make shells, plates, and exoskeletons from calcium minerals. There are thousands of different types of calcifiers—from snails to corals to plankton—dispersed widely throughout the ocean. Early research suggests that acidification may well threaten all of them. When dissolved in water, CO2 forms carbonic acid, which not only eats away at existing calcium-based materials but interferes with the chemical reactions by which new ones are made. INFOGRAPHIC 29.2
Acidic ocean water decreases the ability of aquatic organisms to form shells or exoskeletons, while also dissolving shells and coral that have already been formed.
David Littschwager/National Geographic Creative
An experiment by Orr et al. showed the increased rate of dissolution (dissolving) of pteropod shells in water with less calcium carbonate. In this test, shells were exposed to water containing the lower levels of calcium carbonate we expect to see in 2100 if we continue to use fossil fuels at the same rate that we now do. (At lower pHs, there will be less calcium carbonate in the ocean.)


Explain how the addition of CO2 to ocean water can lead to both the dissolving of existing shells and a reduction in the production of new shells.
When CO2 enters seawater, it is converted to carbonic acid which then releases hydrogen ions (H+). These hydrogen ions can directly dissolve shells when it combines with the calcium carbonate that makes up the shells, separating the calcium from the carbonate part of the molecule. In addition the hydrogen ions can bind to the carbonate in the water, a critical raw material for shell making, rendering it unavailable to organisms that make their shells.
Scientists have already documented significant effects on pteropods—tiny swimming snails that are important food for whales, birds, and fish (including juvenile salmon, pollock, and other commercially important species) in the Arctic and Antarctic. Experiments show that pteropod shells grow more slowly—and even start to dissolve—in acidified seawater. At predicted ocean pHs of the near future, they will become too compromised to support all the life-forms that feed on them.
To be sure, all calcifiers play an important part in the grand choreography of ocean life. But the most substantial of them are coral reefs, like the one Slattery and his team were studying near Aquarius.
coral reef
A large underwater structure formed by colonies of tiny animals (coral) that produce a calcium carbonate exoskeleton that over time build up; found in shallow, warm, tropical seas.
Aquarius is the only facility of its kind: a 73-metric-ton, double-lock pressure vessel, just 13 meters long and about 3 meters wide. That’s big enough to house six people for 8 days, sturdy enough to weather the violent storms that periodically shake the region, and unique enough that for the next week, Slattery and his five fellow aquanauts would be the only people on the planet living at the bottom of the sea.
To a marine biologist, the advantages of this particular perspective are innumerable. Without having to return to the surface every hour, or dive in shifts to complete a mere day’s worth of work, individual team members would be able to take measurements in real time and could observe myriad reef species in all their splendor for hours on end. “Aquarius will enable us to observe and take measurements at much closer intervals than we otherwise might,” Slattery says. “It will give us a much fuller picture of what’s going on down there.”
What he really wanted to get a picture of was the hidden crevices tucked deep within the reef. “These are areas within a coral reef landscape that are naturally acidified,” Slattery explains. “They’re packed with sponges and other ocean life, which means lots of respiration, and at the same time they have poor water circulation.” Because CO2 is released during cellular respiration, Slattery reasoned that CO2 concentrations would be high and pH would be low. (See Chapter 8 for more on cellular respiration.) Slattery and his team hoped the creatures living in such crevices might provide some clues about how the larger reef would respond to an acidified ocean.
KEY CONCEPT 29.2
Acidified ocean water can interfere with shell formation and cause shell breakdown in marine calcifiers; it can also alter the availability of key nutrients.
Once they found these acidified microhabitats, the team planned to measure individual cellular respiration rates for the creatures living in these crevices—a crucial detail that had yet to be ascertained by anyone. “Knowledge of the in situ rates of respiration processes and their impact on local pH has been virtually nonexistent,” says Chris Martens, a marine biologist from the University of North Carolina, Chapel Hill, who has also studied acidification from the Aquarius Reef Base. “But it’s hugely important.” Without these data, researchers can’t tell how much acidification is coming from CO2 absorption from the atmosphere and how much is coming from CO2 respired by the infinite array of ocean life in all the various microenvironments. “We need to know the rough contributions of each before we can possibly hope to develop effective mitigation strategies.”
But first they had to find these acidified microhabitats on the reef itself.