
19.2 Stage One Oxidizes Two Carbon Atoms to Gather Energy-Rich Electrons
✓ 3 Identify the primary catabolic purpose of the citric acid cycle.
✓ 4 Explain the advantage of the oxidation of acetyl CoA in the citric acid cycle.
In the first part of the citric acid cycle, the four-
Citrate Synthase Forms Citrate from Oxaloacetate and Acetyl Coenzyme A
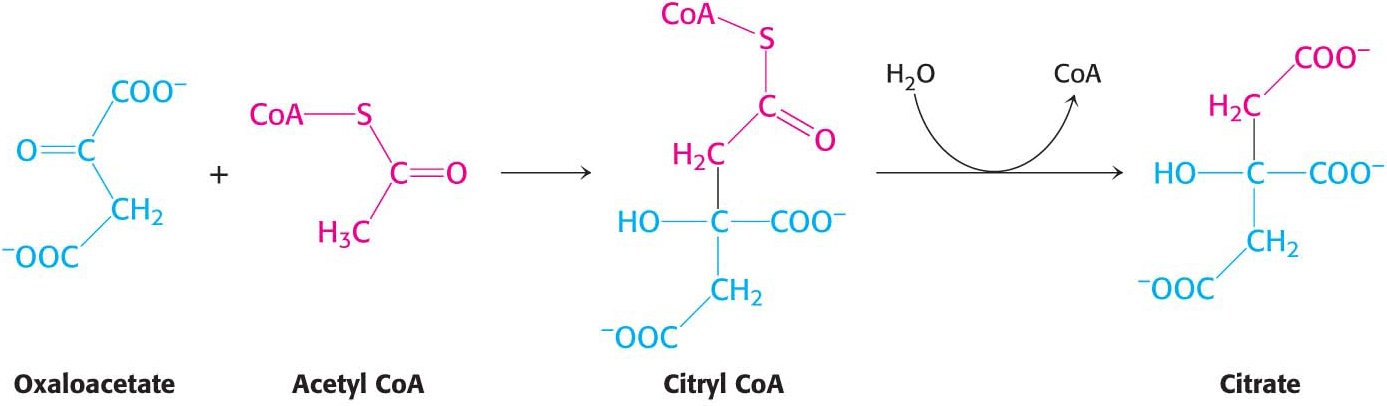
The citric acid cycle begins with the condensation of a four-
DID YOU KNOW?
A synthase is an enzyme that catalyzes a synthetic reaction in which two units are joined usually without the direct participation of ATP (or another nucleoside triphosphate).
This reaction is catalyzed by citrate synthase. Oxaloacetate first condenses with acetyl CoA to form citryl CoA, a molecule that is energy rich because it contains the thioester that originated in acetyl CoA. The hydrolysis of citryl CoA thioester to citrate and CoA drives the overall reaction far in the direction of the synthesis of citrate. In essence,the hydrolysis of the thioester powers the synthesis of a new molecule from two precursors.
The Mechanism of Citrate Synthase Prevents Undesirable Reactions
Because the condensation of acetyl CoA and oxaloacetate initiates the citric acid cycle, side reactions, such as the hydrolysis of acetyl CoA, must be minimized. Let us examine how citrate synthase prevents such wasteful reactions.
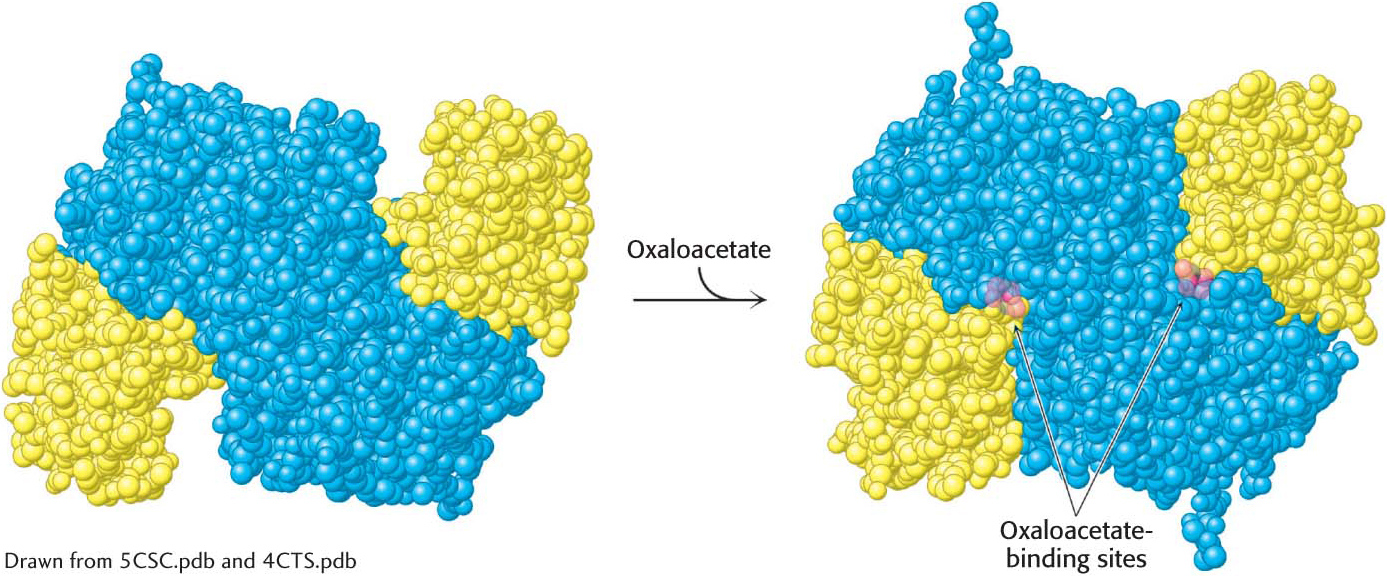
Mammalian citrate synthase is a dimer of identical 49-
DID YOU KNOW?
Citric acid is stored in vacuoles in citrus fruits, where it can sometimes reach a concentration of 0.3 M. It has a tart taste and provides some of the flavor of citrus drinks.
Citrate synthase first catalyzes the condensation of citrate and acetyl CoA to form citryl CoA. The newly formed citryl CoA induces additional structural changes in the enzyme, causing the active site to become completely enclosed. The enzyme then cleaves the citryl CoA thioester by hydrolysis. CoA leaves the enzyme, followed by citrate, and the enzyme returns to the initial open conformation.
We can now understand how the wasteful hydrolysis of acetyl CoA is prevented. Citrate synthase is well suited to the hydrolysis of citryl CoA but not acetyl CoA. How is this discrimination accomplished? First, acetyl CoA does not bind to the enzyme until oxaloacetate is bound and ready for condensation. Second, the catalytic residues crucial for the hydrolysis of the thioester linkage are not appropriately positioned until citryl CoA is formed. Induced fit prevents an undesirable side reaction.
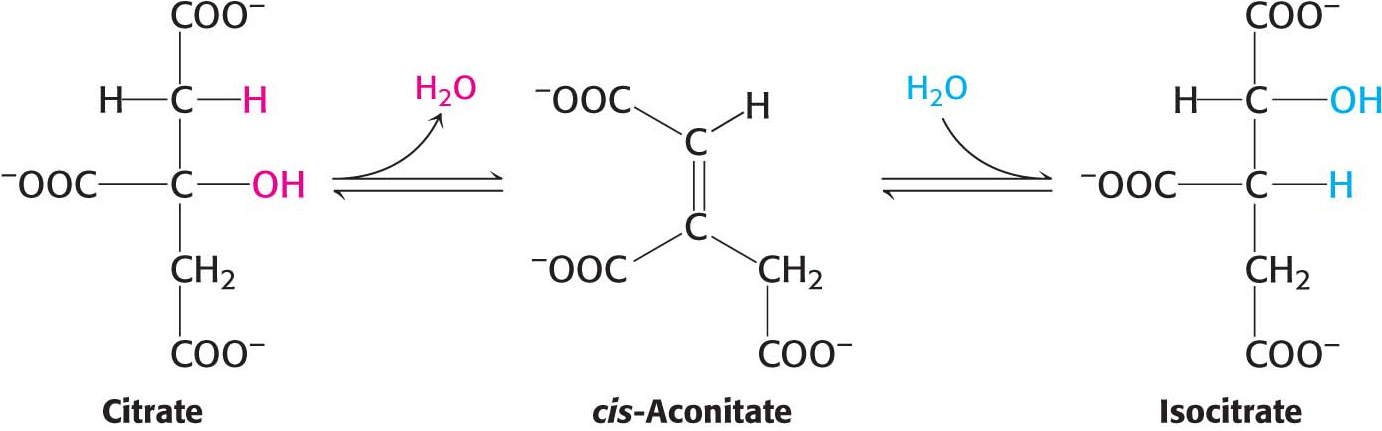
Citrate Is Isomerized into Isocitrate
Keep in mind that a major purpose of the citric acid cycle is the oxidation of carbon atoms leading to the capture of high-
Isocitrate Is Oxidized and Decarboxylated to Alpha-Ketoglutarate
We come now to the first of four oxidation–
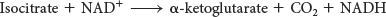
The intermediate in this reaction is oxalosuccinate, an unstable α-ketoacid. While bound to the enzyme, it loses CO2 to form α-ketoglutarate. This oxidation generates the first high-
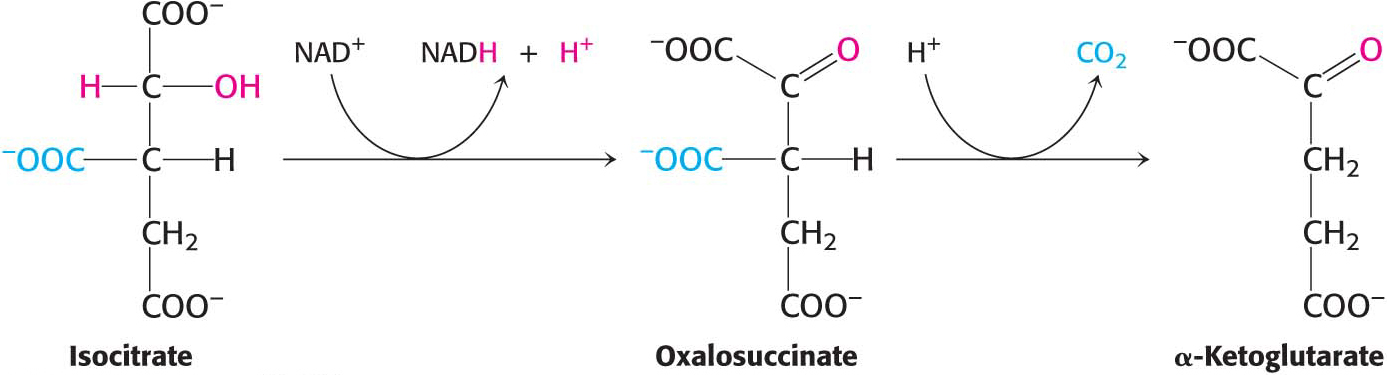
Succinyl Coenzyme A Is Formed by the Oxidative Decarboxylation of Alpha-Ketoglutarate
The conversion of isocitrate into α-ketoglutarate is followed by a second oxidative decarboxylation reaction, removing CO2 from the α-ketoglutarate (five carbon atoms) to form succinyl CoA (four carbon atoms):
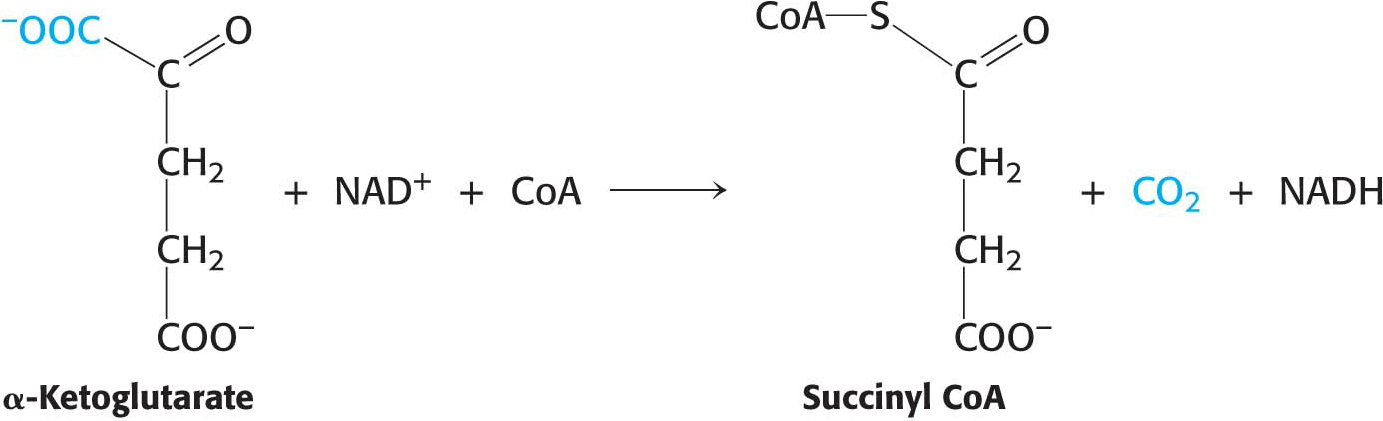
This reaction is catalyzed by the α-ketoglutarate dehydrogenase complex, an organized assembly of three kinds of enzymes that is structurally similar to the pyruvate dehydrogenase complex. In fact, the E3 component is identical in both enzymes. The oxidative decarboxylation of α-ketoglutarate closely resembles that of pyruvate, also an α-ketoacid:
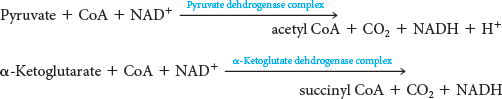
Both reactions include the decarboxylation of an α-ketoacid and the subsequent formation of a thioester linkage with CoA that has a high transfer potential. The reaction mechanisms are entirely analogous, employing the same coenzymes and reaction steps. At this point in the citric acid cycle, two carbon atoms have entered the cycle and two carbon atoms have been oxidized to CO2. The electrons from the oxidations are captured in two molecules of NADH.
