16-1 The Sun’s energy is generated by thermonuclear reactions in its core
 The Sun is the largest member of the solar system. It has almost a thousand times more mass than all the planets, moons, asteroids, comets, and meteoroids put together. But the Sun is also a star. In fact, it is a remarkably typical star, with a mass, size, surface temperature, and chemical composition that are roughly midway between the extremes exhibited by the myriad other stars in the heavens. Table 16-1 lists essential data about the Sun.
The Sun is the largest member of the solar system. It has almost a thousand times more mass than all the planets, moons, asteroids, comets, and meteoroids put together. But the Sun is also a star. In fact, it is a remarkably typical star, with a mass, size, surface temperature, and chemical composition that are roughly midway between the extremes exhibited by the myriad other stars in the heavens. Table 16-1 lists essential data about the Sun.
TABLE 16-1 Sun Data

Solar Energy
The Sun’s surface is hot enough to emit visible light
For most people, what matters most about the Sun is the energy that it radiates into space. With-out the Sun’s warming rays, our oceans would freeze. Furthermore, since most life is part of a food chain dependent on sunlight for energy, an Earth without sunlight would be nearly dead. To understand the nature of life on Earth, we must understand the Sun.
Why is the Sun such a strong source of energy? Recall that the hotter an object, the more light it emits (see Figure 5-9). The Sun’s spectrum is close to that of an idealized blackbody with a temperature of 5800 K (see Section 5-3 and Section 5-4, especially Figure 5-13). Thanks to this high temperature, each square meter of the Sun’s surface emits a tremendous amount of radiation, principally at visible wavelengths. Indeed, the Sun is the only object in the solar system that emits substantial amounts of visible light. The surface temperatures of the Moon and planets are very low compared to the Sun; the visible light that we see from bodies in the solar system is actually sunlight that struck those worlds and was reflected toward Earth.
The bright Sun emits a tremendous amount of energy. The total amount of energy emitted by the Sun each second, called its luminosity, is about 3.9 × 1026 watts (3.9 × 1026 joules of energy emitted per second). (We discussed the relationship between the Sun’s surface temperature, radius, and luminosity in Box 5-2.) Astronomers denote the Sun’s luminosity by the symbol L⊙. A circle with a dot in the center is the astronomical symbol for the Sun and was also used by ancient astrologers.
Does the Sun emit so much energy because it is hot or because it is big? To put the Sun’s temperature and size into perspective, we can make a comparison with ovens and lightbulbs. The solar surface is about 10 times hotter than the maximum temperature of a household oven. A factor of 10 might not sound like a great amount between the Sun and an oven, but we saw in Section 5-4, for the Stefan-Boltzmann law, F (energy flux) = σT4, that raising an object’s temperature 10 times will increase the energy it emits per second by a factor of 10,000! But the Sun is also much bigger than an oven, and to appreciate the effect of size, let us consider lightbulbs.
Suppose you stood in a room that contained an object at the same temperature as the Sun but with only a surface area of 1 square millimeter. Would it burn you up? No. Such an object would emit around 100 watts, similar to a household lightbulb. Just like a lightbulb, you would have to put your hand quite close to feel any warmth. What helps make the Sun so strong is that each square meter patch of the solar surface emits about as much as a million 100-watt lightbulbs, and the Sun’s surface area has more than a million trillion of these 1-square-meter patches. Taken together, it is the high temperature and large size of the Sun that make it so bright.
CONCEPT CHECK 16-1
The Sun emits light at nearly all possible wavelengths. At which range of wavelengths is most of the Sun’s energy emitted?
The Sun emits most of its energy in the form of visible light.
The Source of the Sun’s Energy: Early Ideas
The bright Sun leads us to a more fundamental question: What keeps the Sun’s visible surface so hot? Or, put another way, what is the fundamental source of the tremendous energy that the Sun radiates into space? For centuries, this question was one of the greatest mysteries in science. The mystery deepened in the nineteenth century, when geologists and biologists found convincing evidence that life has existed on Earth for at least several hundred million years. Since life as we know it depends crucially on sunlight, the conclusion was that the Sun was at least several hundred million years old. (We now know that Earth is 4.54 billion years old and that life has existed on it for most of its history.) The source of the Sun’s energy posed a severe problem for physicists. What source of energy could have kept the Sun shining for so long (Figure 16-1)?

The Sun The Sun’s visible surface has a temperature of about 5800 K. At this temperature, all solids and liquids vaporize to form gases. It was only in the twentieth century that scientists discovered what has kept the Sun so hot for billions of years—the thermonuclear fusion of hydrogen nuclei in the Sun’s core.
One attempt to explain solar energy was made in the mid-1800s by the English physicist Lord Kelvin (for whom the temperature scale is named) and the German scientist Hermann von Helmholtz. They argued that the tremendous weight of the Sun’s outer layers should cause the Sun to contract gradually, compressing its interior gases. Whenever a gas is compressed, its temperature rises. (You can demonstrate this with a bicycle pump: As you pump air into a tire, the temperature of the air increases and the pump becomes warm to the touch.) Kelvin and Helmholtz thus suggested that gravitational contraction could cause the Sun’s gases to become hot enough to radiate energy out into space.
This process, called Kelvin-Helmholtz contraction, actually does occur during the earliest stages of the birth of a star like the Sun (see Section 8-4). But Kelvin-Helmholtz contraction cannot be the major source of the Sun’s energy today. If it were, the Sun would have had to be much larger in the relatively recent past. Helmholtz’s own calculations showed that the Sun could have started its initial collapse from the solar nebula no more than about 25 million years ago. But the geological and fossil record shows that Earth is far older than that, and so the Sun must be as well. Hence, this model of a Sun that shines because it shrinks cannot be correct.
On Earth, a common way to produce heat and light is by burning fuel, such as a log in a fireplace or charcoal in a barbeque grill. Is it possible that a similar process explains the energy released by the Sun? The answer is no, because this process could not continue for a long enough time to explain the age of Earth. It might seem difficult to estimate how long the Sun could last if its energy came from burning, but the calculation only requires a few steps. To begin with, the chemical reactions involved in burning release roughly 10−19 joule of energy per atom. Therefore, the number of atoms that would have to undergo chemical reactions each second to generate the Sun’s luminosity of 3.9 × 1026 joules per second is approximately
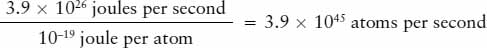
From its mass and chemical composition, we know that the Sun contains about 1057 atoms. Thus, the length of time that would be required to consume the entire Sun by burning is
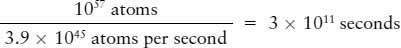
There are about 3 × 107 seconds in a year. Hence, in this model, the Sun would burn itself out in a mere 10,000 (104) years! This period of time is far shorter than the known age of Earth, so chemical reactions cannot explain how the Sun shines. This is a very powerful result because even though there are numerous types of chemical reactions that release energy (anything from fire to dynamite), the maximum possible chemical energy in the Sun’s total mass could not keep it burning for very long.
The Source of the Sun’s Energy: Discovering Thermonuclear Fusion
The source of the Sun’s luminosity could be explained if there were a process that was like burning but released much more energy per atom. Then the rate at which atoms would have to be consumed would be far less, and the lifetime of the Sun could be long enough to be consistent with the known age of Earth. Albert Einstein discovered the key to such a process in 1905. According to his special theory of relativity, a quantity m of mass can in principle be converted into an amount of energy E according to a now-famous equation:
Einstein’s mass-energy equation
E = mc2
- m = quantity of mass, in kg
- c = speed of light = 3 × 108 m/s
- E = amount of energy into which the mass can be converted, in joules
The speed of light c is a large number, so c2 is huge. Therefore, a small amount of matter can release an awesome amount of energy. For example, if the mass in just one penny (about 2.5 grams) were completely converted into energy, it would release about 5 times the energy of the nuclear bomb detonated over Hiroshima, Japan, during World War II. (While the energy in a penny’s worth of mass is impressive, the largest nuclear bombs release the energy contained in the mass of 10 rolls of quarters; see Figure 1-6.)
Inspired by Einstein’s ideas, astronomers began to wonder if the Sun’s energy output might come from the conversion of matter into energy. The Sun’s low density of 1410 kg/m3 indicates that it must be made of the very lightest atoms, primarily hydrogen and helium. In the 1920s, the British astronomer Arthur Eddington showed that temperatures near the center of the Sun must be so high that atoms become completely ionized. Hence, at the Sun’s center we expect to find hydrogen nuclei and electrons flying around independent of each other.
Ideas from relativity and nuclear physics led to an understanding of how the Sun shines
Another British astronomer, Robert Atkinson, suggested that under these conditions hydrogen nuclei could fuse together to produce helium nuclei in a nuclear reaction that transforms a tiny amount of mass into a large amount of energy. Experiments in the laboratory using individual nuclei show that such reactions can indeed take place. The process of converting hydrogen into helium is called hydrogen fusion. (It is also sometimes called hydrogen burning, even though nothing is actually burned in the conventional sense. Ordinary burning involves chemical reactions that rearrange the outer electrons of atoms but have no effect on the atoms’ nuclei.)
The fusing together of nuclei is also called thermonuclear fusion, because it can take place only at extremely high temperatures. This is because all nuclei have a positive electric charge and so tend to repel one another. But in the extreme heat and pressure at the Sun’s center, hydrogen nuclei (protons) are moving so fast that they can overcome their electric repulsion and actually touch one another. When that happens, thermonuclear fusion can take place.
ANALOGY
You can think of protons as tiny electrically charged spheres that are coated with a very powerful glue. If the spheres are not touching, the repulsion between their charges pushes them apart. But if the spheres are forced into contact, the strength of the glue “fuses” them together. In this fusion, a little bit of mass is lost, which gets converted into energy.
CAUTION!
Be careful not to confuse thermonuclear fusion with the similar-sounding process of nuclear fission. In nuclear fusion, energy is released by joining or fusing together nuclei of lightweight atoms such as hydrogen. In nuclear fission, by contrast, the nuclei of very massive atoms such as uranium or plutonium release energy by fragmenting into smaller nuclei. Nuclear power plants produce energy using fission, not fusion. (Generating power using fusion has been a goal of researchers for decades, but no one has yet devised a commercially viable way to do this.)
CONCEPT CHECK 16-2
If hydrogen nuclei are positively charged, under what conditions can two hydrogen nuclei overcome the repulsive force of their electric charges and fuse together, thus releasing energy according to Einstein’s equation, E = mc2?
At the extremely high temperatures and pressures existing in the Sun’s core, hydrogen nuclei can move fast enough to overcome the repulsive force from their positive electric charges and fuse together.
CALCULATION CHECK 16-1
How much energy is released when just 5 kg of mass is converted into energy?
According to Einstein’s equation that E = mc2, a mass of 5 kg is equivalent to 5 kg × (3 × 108 m/s)2 = 45 × 1016 joules, which is equivalent to burning about 100,000 metric tons of coal!
Converting Hydrogen to Helium
We learned in Section 5-8 that the nucleus of a hydrogen atom (H) consists of a single proton. The nucleus of a helium atom (He) consists of two protons and two neutrons. In the nuclear process that Atkinson described, four hydrogen nuclei combine to form one helium nucleus, with a concurrent release of energy:
4 H → He + energy
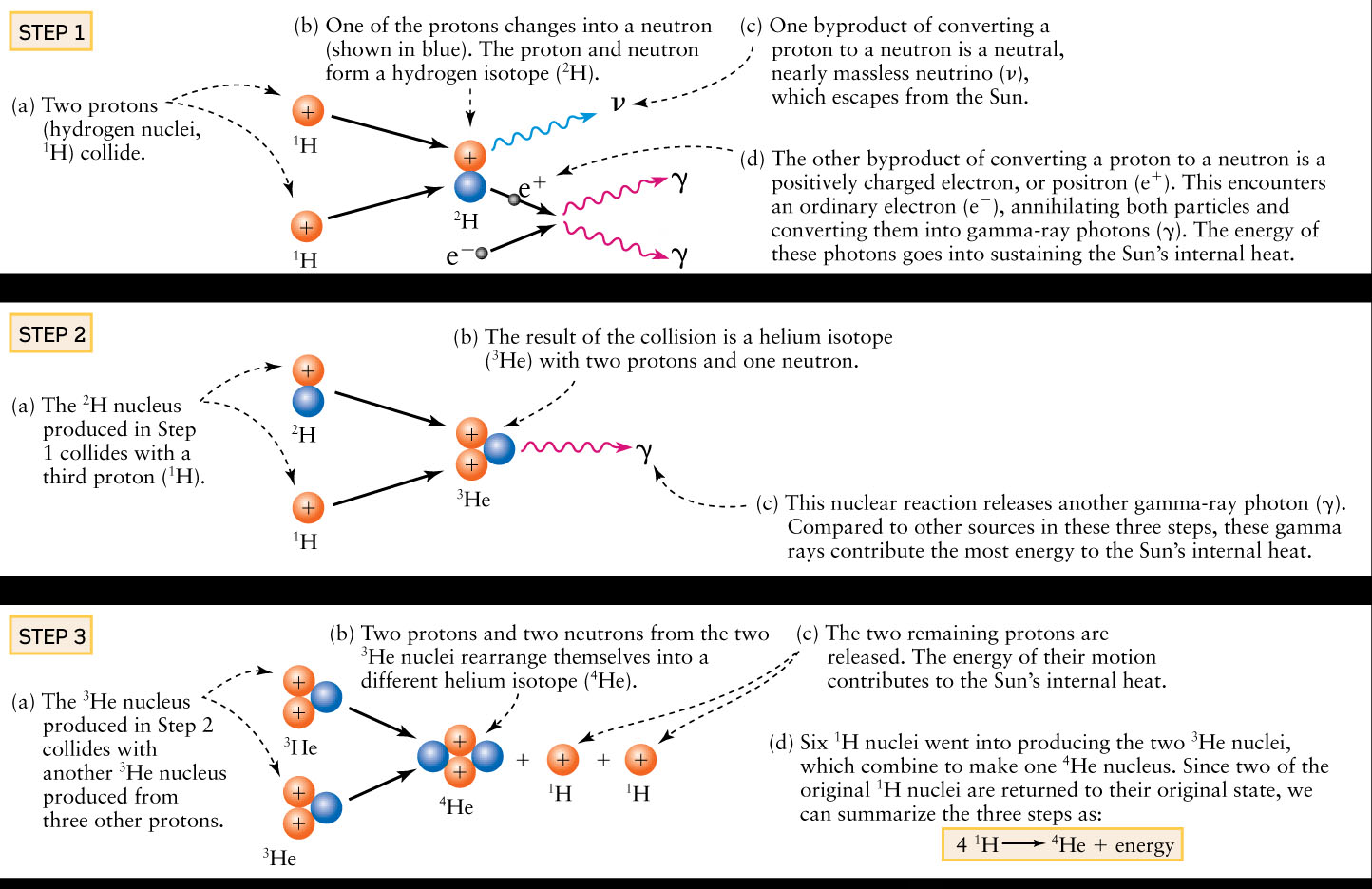
In several separate reactions, two of the four protons are changed into neutrons, and eventually combine with the remaining protons to produce a helium nucleus. This sequence of reactions is called the proton-proton chain. The Cosmic Connections figure depicts the proton-proton chain in detail.
Each time this process takes place, a small fraction (0.7%) of the initial combined mass of the hydrogen nuclei does not show up in the final mass of the helium nucleus. This “lost” mass is converted into energy. Box 16-1 describes how to use Einstein’s mass-energy equation to calculate the amount of energy released.
COSMIC CONNECTIONS
The Proton–Proton Chain
The most common form of hydrogen fusion in the Sun involves three steps, each of which releases energy.


BOX 16-1 TOOLS OF THE ASTRONOMER’S TRADE
Converting Mass into Energy
The Cosmic Connections: The Proton-Proton Chain figure shows the steps involved in the thermonuclear fusion of hydrogen at the Sun’s center. In these steps, four protons are converted into a single nucleus of 4He, the most common isotope of helium with two protons and two neutrons. (As we saw in Box 5-5, different isotopes of the same element have the same number of protons but different numbers of neutrons.) The reaction depicted in the Cosmic Connections Step 1 also produces a neutral, nearly massless particle called the neutrino. Neutrinos respond hardly at all to ordinary matter, so they travel almost unimpeded through the Sun’s massive bulk. Hence, the energy that neutrinos carry is quickly lost into space. This loss is not great, however, because the neutrinos carry relatively little energy. (See Section 16-4 for more about these curious particles.)
Most of the energy released by thermonuclear fusion appears in the form of gamma-ray photons. The energy of these photons remains trapped within the Sun for a long time, thus maintaining the Sun’s intense internal heat. Some gamma-ray photons are produced by the reaction shown as Step 2 in the Cosmic Connections figure. Others appear when an electron in the Sun’s interior annihilates a positively charged electron, or positron, which is a by-product of the reaction shown in Step 1 in the Cosmic Connections figure. An electron and a positron are respectively matter and antimatter, and they convert their mass entirely into energy when they meet. (You may have thought that “antimatter” was pure science fiction. In fact, tremendous amounts of antimatter are being created and annihilated in the Sun as you read these words.)
We can summarize the thermonuclear fusion of hydrogen as follows:
4 1H → 4He + neutrinos + gamma-ray photons
To calculate how much energy is released in this process, we use Einstein’s mass-energy formula: The energy released is equal to the amount of mass consumed multiplied by c2, where c is the speed of light. To see how much mass is consumed, we compare the combined mass of four hydrogen atoms (the ingredients) to the mass of one helium atom (the product):
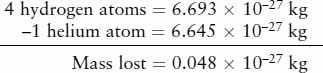
Thus, a small fraction (0.7%) of the mass of the hydrogen going into the nuclear reaction does not show up in the mass of the helium. This lost mass is converted into an amount of energy E = mc2:
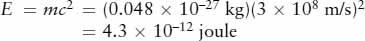
This amount of energy is released by the formation of a single helium atom. It would light a 10-watt lightbulb for almost one-half of a trillionth of a second.
EXAMPLE: How much energy is released when 1 kg of hydrogen is converted to helium?
Situation: We are given the initial mass of hydrogen. We know that a fraction of the mass is lost when the hydrogen undergoes fusion to make helium; our goal is to find the quantity of energy into which this lost mass is transformed.
Tools: We use the equation E = mc2 and the result that 0.7% of the mass is lost when hydrogen is converted into helium.
Answer: When 1 kg of hydrogen is converted to helium, the amount of mass lost is 0.7% of 1 kg, or 0.007 kg. (This means that 0.993 kg of helium is produced.) Using Einstein’s equation, we find that this missing 0.007 kg of matter is transformed into an amount of energy equal to
E = mc2 = (0.007 kg)(3 × 108 m/s)2 = 6.3 × 1014 joules
Review: The energy released by the fusion of 1 kg of hydrogen is the same as that released by burning 20,000 metric tons (2 × 107 kg) of coal! Hydrogen fusion is a much more efficient energy source than ordinary burning.
The Sun’s luminosity is 3.9 × 1026 joules per second. To generate this much power, hydrogen must be consumed at a rate of

That is, the Sun converts 600 million metric tons of hydrogen into helium every second.
CAUTION!
You may have heard the idea that mass is always conserved (that is, it is neither created nor destroyed), or that energy is always conserved in a reaction. Einstein’s ideas show that neither of these statements is quite correct, because mass can be converted into energy and vice versa. A more accurate statement is that the total amount of mass plus energy is conserved. Hence, the destruction of mass in the Sun does not violate any laws of nature.
For every four hydrogen nuclei converted into a helium nucleus, 4.3 × 10−12 joule of energy is released—only enough energy to power a small Christmas light for a trillionth of a second. This amount of energy may seem tiny, but it is about 107 times larger than the amount of energy released in a typical chemical reaction, such as occurs in ordinary burning. Thus, thermonuclear fusion explains how the Sun could have been shining for billions of years.
To produce the Sun’s luminosity of 3.9 × 1026 joules per second, 6 × 1011 kg (600 million metric tons) of hydrogen must be converted into helium each second—this is about 100 times the mass of Egypt’s largest pyramid. This rate is prodigious, but there is, literally, an astronomical amount of hydrogen in the Sun. In particular, the Sun’s core contains enough hydrogen to have been giving off energy at the present rate for as long as the solar system has existed, about 4.56 billion years, and to continue doing so for more than 6 billion years into the future.
 The proton-proton chain is also the energy source for many of the stars in the sky. In stars with central temperatures that are much hotter than that of the Sun, however, hydrogen fusion proceeds according to a different set of nuclear reactions, called the CNO cycle, in which carbon, nitrogen, and oxygen nuclei absorb protons to produce helium nuclei. Still other thermonuclear reactions, such as helium fusion, carbon fusion, and oxygen fusion, occur late in the lives of many stars.
The proton-proton chain is also the energy source for many of the stars in the sky. In stars with central temperatures that are much hotter than that of the Sun, however, hydrogen fusion proceeds according to a different set of nuclear reactions, called the CNO cycle, in which carbon, nitrogen, and oxygen nuclei absorb protons to produce helium nuclei. Still other thermonuclear reactions, such as helium fusion, carbon fusion, and oxygen fusion, occur late in the lives of many stars.
CONCEPT CHECK 16-3
If 1 kg of hydrogen combines to form helium in the proton-proton chain, why is only 0.007 kg (0.7%) available to be converted into energy?
When 1 kg of hydrogen combines to form helium, the vast majority of the mass ends up in helium atoms, with only 0.7% of the original mass converted into energy.
CONCEPT CHECK 16-4
How do astronomers estimate that our Sun can continue to shine for more than 6 billion years?
Astronomers use the current energy output of the Sun to estimate how fast the Sun is consuming its usable fuel (hydrogen). Then, the amount of hydrogen fuel remaining in the Sun’s core indicates how much longer the Sun will shine.
