9-5 Earth’s atmosphere has changed substantially over our planet’s history
The composition of our atmosphere has been dramatically altered by the evolution of life
Both plate tectonics and Earth’s magnetic field paint a picture of a planet with a dynamic, evolving surface and interior. Our atmosphere also has changed and evolved substantially over Earth’s 4.54-billion-year history. As we will see, this evolution explains why we have an atmosphere totally unlike that of any other world in the solar system.
Earth’s Early Atmosphere
When Earth first formed by accretion of planetesimals (see Section 8-5), gases were probably trapped within Earth’s interior in the same proportions that they were present in the solar nebula. But since the early Earth was hot enough to be molten throughout its volume, most of these trapped gases were released. As we discussed in Section 7-4 and Box 7-2, Earth’s gravity was too weak to prevent hydrogen and helium—the two most common kinds of atoms in the universe, but also the least massive—from leaking away into space. The atmosphere that remained still contained substantial amounts of hydrogen, but in the form of relatively massive molecules of water vapor (H2O). In fact, water vapor was probably the dominant constituent of the early atmosphere, which is thought to have been about 100 times denser than our present-day atmosphere.
In addition to releasing water, intense volcanic activity of this early period would have released carbon dioxide (CO2) and ammonia (NH3). This process, in which volcanoes inject the atmosphere with gas, is called outgassing. As Earth cooled, much of the water condensed and fell as rain. But the rain lowered carbon dioxide levels as well.
Carbon dioxide dissolves in rainwater and the oceans, where it combines with other substances to form a class of minerals called carbonates. (Limestone and marble are examples of carbonate-bearing rock.) These form sediments on the ocean floor, which are eventually recycled into the crust by subduction. As an example, marble (Figure 9-18c) is a metamorphic rock formed deep within the crust from limestone, a carbonate-rich sedimentary rock.
At the same time that levels of water and carbon dioxide were decreasing, ultraviolet sunlight broke ammonia apart to create two gases in the atmosphere: hydrogen (H2) and nitrogen (N2). The hydrogen, being too light, escaped into space. Through the loss or relocation of water vapor, carbon dioxide, and hydrogen, the remaining nitrogen became the dominant component in the atmosphere. Today, nitrogen still forms the bulk of our atmosphere—about 78%—but it is the other main component that matters the most—oxygen.
Life’s Impact on Earth’s Atmosphere
The appearance of life on Earth set into motion a radical transformation of the atmosphere. Early single-cell organisms converted energy from sunlight into chemical energy using photosynthesis, a chemical process that consumes CO2 and water and releases oxygen (O2). Oxygen molecules are very reactive, so originally most of the O2 produced by photosynthesis combined with other substances to form minerals called oxides. (Evidence for this can be found in rock formations of various ages. The oldest rocks have very low oxide content, while oxides are prevalent in rocks that formed after the appearance of organisms that used photosynthesis.) But as life proliferated, the amount of photosynthesis increased dramatically. Eventually, so much oxygen was being produced that it could not all be absorbed to form oxides, and O2 began to accumulate in the atmosphere. Figure 9-23 shows how the amount of O2 in the atmosphere has increased over the history of Earth.
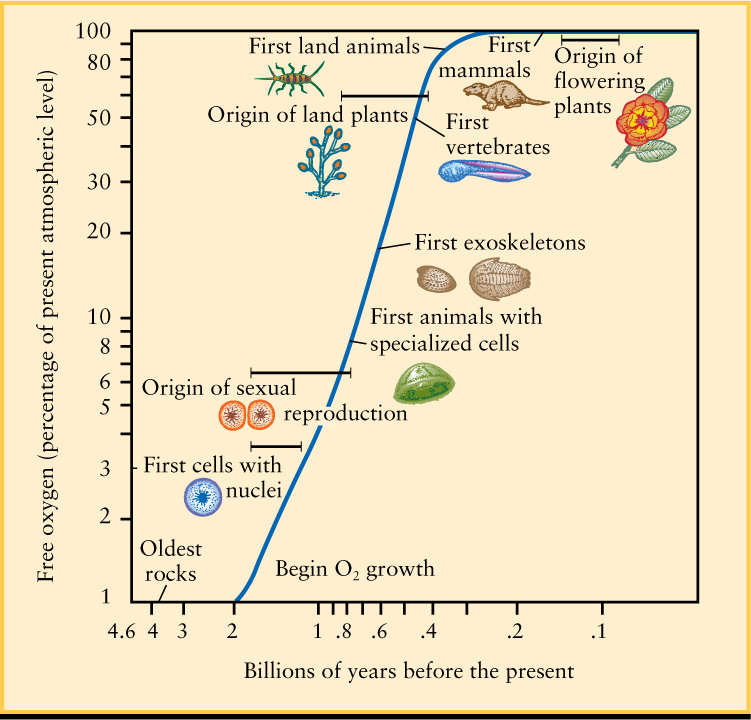
About 2 billion (2 × 109) years ago, a new type of life evolved to take advantage of the newly abundant oxygen. These new organisms produced energy by consuming oxygen and releasing carbon dioxide—a process called respiration that is used by all modern animals, including humans. Such organisms thrived because photosynthetic plants continued to add even more oxygen to the atmosphere. The abundance of atmospheric oxygen is due almost exclusively to the presence of life—a situation that has no parallel anywhere else in the solar system. For this reason, finding an oxygen atmosphere around a planet in another solar system would signal the possibility of life beyond Earth. And, as we saw in Section 8-7, determining the composition of atmospheres around extrasolar planets is now possible.
Comparing Atmospheres: Earth, Venus, and Mars
Table 9-4 shows the dramatic differences between Earth’s atmosphere and those of Venus and Mars. (Mercury, the other terrestrial planet, is too small and has too little gravity to hold an appreciable atmosphere.) The greater intensity of sunlight on Venus caused higher temperatures, which boiled any liquid water and made it impossible for CO2 to be taken out of the atmosphere and put back into rocks. Venus’s atmosphere thus became far denser than our own and rich in greenhouse gases. The result was a very strong greenhouse effect that raised temperatures on Venus to their present value of about 460°C (733 K = 855°F).
| Venus | Earth | Mars | |
|---|---|---|---|
| Nitrogen (N2) | 3.5% | 78.08% | 2.7% |
| Oxygen (O2) | almost zero | 20.95% | almost zero |
| Carbon dioxide (CO2) | 96.5% | 0.035% | 95.3% |
| Water vapor (H2O) | 0.003% | about 1% | 0.03% |
| Other gases | almost zero | almost zero | 2% |
Just the opposite happened on Mars, where sunlight is less than half as intense as it is on Earth. The lower temperatures drove CO2 from the atmosphere into Martian rocks and froze groundwater to considerable depths beneath the planet’s surface. The atmosphere that remains on Mars has a similar composition to that of Venus but is less than 1/10,000 as dense. On neither Venus nor Mars was life able to blossom and transform the atmosphere as it did here on Earth.
We have thus uncovered a general rule about the terrestrial planets:
The closer a terrestrial planet is to the Sun, the stronger the greenhouse effect, and the higher the planet’s surface temperature.
This rule suggests that if Earth had formed a bit closer to or farther from the Sun, temperatures on our planet might have been too high or too low for life ever to evolve. Thus, our very existence is a result of Earth’s special position in the solar system.
CONCEPT CHECK 9-7
Which, if any, of the two most abundant gases in Earth’s present atmosphere came directly from volcanoes?
Nitrogen and oxygen are the most abundant gases in our atmosphere, but neither came directly form volcanoes. Ammonia (NH3) came directly from volcanoes, but after sunlight broke this gas apart, only nitrogen gas remained. Later, photosynthesizing microorganisms produced oxygen.