12.6
Eukaryotic Cells Contain Compartments Bounded by Internal Membranes
Thus far, we have considered only the plasma membrane of cells. Some bacteria and archaea have only this single membrane, surrounded by a cell wall. Other bacteria, such as E. coli, have two membranes separated by a cell wall (made of proteins, peptides, and carbohydrates) lying between them (Figure 12.34). The inner membrane acts as the permeability barrier, and the outer membrane and the cell wall provide additional protection. The outer membrane is quite permeable to small molecules, owing to the presence of porins. The region between the two membranes containing the cell wall is called the periplasm.
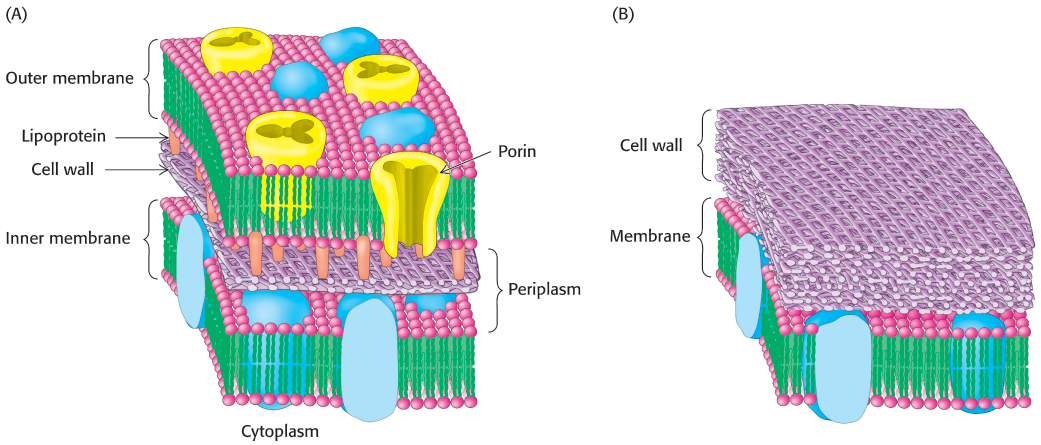
FIGURE 12.34Cell membranes of prokaryotes. A schematic view of the membrane of bacterial cells surrounded by (A) two membranes or (B) one membrane.
Eukaryotic cells, with the exception of plant cells, do not have cell walls, and their cell membranes consist of a single lipid bilayer. In plant cells, the cell wall is on the outside of the plasma membrane. Eukaryotic cells are distinguished from prokaryotic cells by the presence of membranes inside the cell that form internal compartments. For example, peroxisomes, organelles that play a major role in the oxidation of fatty acids for energy conversion, are defined by a single membrane. Mitochondria, the organelles in which ATP is synthesized, are surrounded by two membranes. As in the case for a bacterium, the outer membrane is quite permeable to small molecules, whereas the inner membrane is not. Indeed, considerable evidence now indicates that mitochondria evolved from bacteria by endosymbiosis (Section 18.1). The nucleus is also surrounded by a double membrane, the nuclear envelope, that consists of a set of closed membranes that come together at structures called nuclear pores (Figure 12.35). These pores regulate transport into and out of the nucleus. The nuclear envelope is linked to another membrane-defined structure, the endoplasmic reticulum, which plays a host of cellular roles, including drug detoxification and the modification of proteins for secretion. Thus, a eukaryotic cell contains interacting compartments, and transport into and out of these compartments is essential to many biochemical processes.
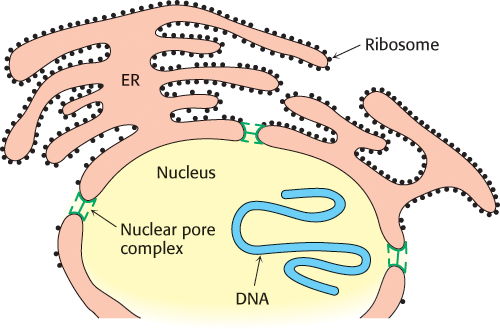
FIGURE 12.35Nuclear envelope. The nuclear envelope is a double membrane connected to another membrane system of eukaryotes, the endoplasmic reticulum. [Information from E. C. Schirmer and L. Gerace, Genome Biol. 3(4):1008.1–1008.4, 2002, reviews, Fig.1.]
Membranes must be able to separate or join together so that cells and compartments may take up, transport, and release molecules. Many cells take up molecules through the process of receptor-mediated endocytosis. Here, a protein or larger complex initially binds to a receptor on the cell surface. After the receptor is bound, specialized proteins act to cause the membrane in this region to invaginate. One of these specialized proteins is clathrin, which polymerizes into a lattice network around the growing membrane bud, often referred to as a clathrin-coated pit (Figure 12.36). The invaginated membrane eventually breaks off and fuses to form a vesicle. Various hormones, transport proteins, and antibodies employ receptor-mediated endocytosis to gain entry into a cell. A less-advantageous consequence is that this pathway is available to viruses and toxins as a means of invading cells. The reverse process—the fusion of a vesicle to a membrane—is a key step in the release of neurotransmitters from a neuron into the synaptic cleft (Figure 12.37).
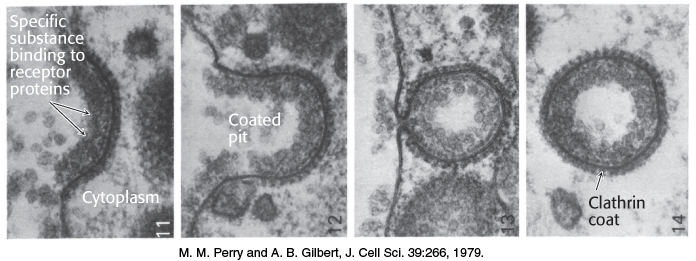
FIGURE 12.36Vesicle formation by receptor-mediated endocytosis. Receptor binding on the surface of the cell induces the membrane to invaginate, with the assistance of specialized intracellular proteins such as clathrin. The process results in the formation of a vesicle within the cell.
[M. M. Perry and A. B. Gilbert, J. Cell Sci. 39:266, 1979.]
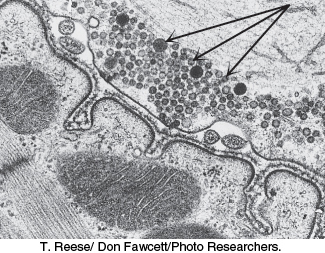
FIGURE 12.37Neurotransmitter release. Neurotransmitter-containing synaptic vesicles (indicated by the arrows) are arrayed near the plasma membrane of a nerve cell. Synaptic vesicles fuse with the plasma membrane, releasing the neurotransmitter into the synaptic cleft.
[T. Reese/Don Fawcett/Photo Researchers.]
Let us consider one example of receptor-mediated endocytosis. Iron is a critical element for the function and structure of many proteins, including hemoglobin and myoglobin (Chapter 7). However, free iron ions are highly toxic to cells, owing to their ability to catalyze the formation of free radicals. Hence, the transport of iron atoms from the digestive tract to the cells where they are most needed must be tightly controlled. In the bloodstream, iron is bound very tightly by the protein transferrin, which can bind two Fe3+ ions with a dissociation constant of 10−23 M at neutral pH. Cells requiring iron express the transferrin receptor in their plasma membranes (Section 32.4). Formation of a complex between the transferrin receptor and iron-bound transferrin initiates receptor-mediated endocytosis, internalizing these complexes within vesicles called endosomes (Figure 12.38). As the endosomes mature, proton pumps within the vesicle membrane lower the lumenal pH to about 5.5. Under these conditions, the affinity of iron ions for transferrin is reduced; these ions are released and are free to pass through channels in the endosomal membranes into the cytoplasm. The iron-free transferrin complex is recycled to the plasma membrane, where transferrin is released back into the bloodstream and the transferrin receptor can participate in another uptake cycle.
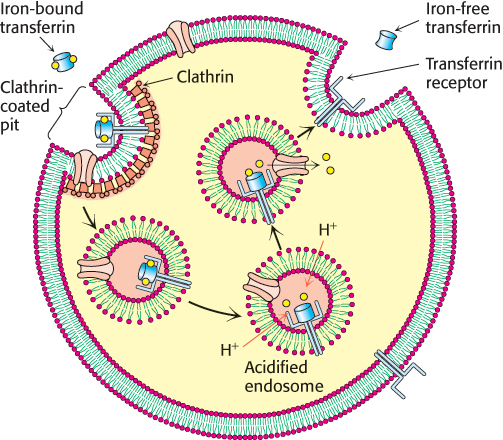
FIGURE 12.38The transferrin receptor cycle. Iron-bound transferrin binds to the transferrin receptor (TfR) on the surface of cells. Receptor-mediated endocytosis occurs, leading to the formation of a vesicle called an endosome. As the lumen of the endosome is acidified by the action of proton pumps, iron is released from transferrin, passes through channels in the membrane, and is utilized by the cell. The complex between iron-free transferrin and the transferrin receptor is returned to the plasma membrane for another cycle.
[Information from L. Zecca et al., Nat. Rev. Neurosci. 5:863–873, 2004, Fig.1.]
Although budding and fusion appear deceptively simple, the structures of the intermediates in these processes and the detailed mechanisms remain ongoing areas of investigation. These processes must be highly specific in order to prevent incorrect membrane fusion events. SNARE (soluble N-ethylmaleimide-sensitive-factor attachment protein receptor) proteins from both membranes help draw appropriate lipid bilayers together by forming tightly coiled four-helical bundles (Figure 12.39). Once these membranes are in close apposition, the fusion process can proceed. SNARE proteins, encoded by gene families in all eukaryotic cells, largely determine the compartment with which a vesicle will fuse. The specificity of membrane fusion ensures the orderly trafficking of membrane vesicles and their cargos through eukaryotic cells.
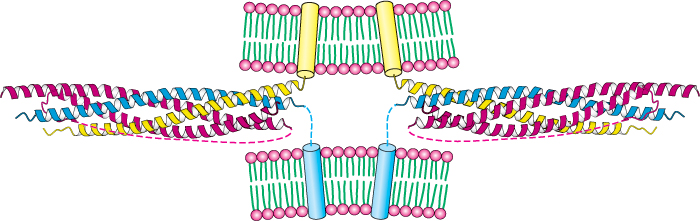
FIGURE 12.39SNARE complexes initiate membrane fusion. The SNARE protein synaptobrevin (yellow) from one membrane forms a tight four-helical bundle with the corresponding SNARE proteins syntaxin-1 (blue) and SNAP25 (red) from a second membrane. The complex brings the membranes close together, initiating the fusion event.
[Drawn from 1SFC.pdb.]





