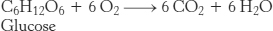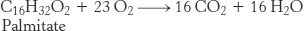Mitochondrial biogenesis is stimulated by muscular activity
When muscle is stimulated to contract during exercise by receiving nerve impulses from motor neurons, calcium is released from the sarcoplasmic reticulum. Calcium induces muscle contraction, as will be discussed in Chapter 35. Recall that calcium is also a potent second messenger and frequently works in association with the calcium-binding protein calmodulin (Section 14.1). In its capacity as a second messenger, calcium stimulates various calcium-dependent enzymes, such as calmodulin-dependent protein kinase. The calcium-dependent enzymes, as well as AMPK, subsequently activate particular transcription-factor complexes. As we will see in Chapters 29 and 31, transcription factors are proteins that control gene expression. Two patterns of gene expression, in particular, change in response to regular exercise (Figure 27.10). Regular exercise enhances the production of proteins required for fatty acid metabolism, such as the enzymes of β oxidation. Interestingly, fatty acids themselves function as signal molecules to activate the transcription of enzymes of fatty acid metabolism. Additionally, another set of transcription factors activated by the calcium signal cascade initiates a signal pathway that increases mitochondrial biogenesis. In concert, the increase in fatty acid oxidizing capability and additional mitochondria allow for the efficient metabolism of fatty acids. Because an excess of fatty acids results in insulin resistance, as already discussed, efficient metabolism of fatty acids results in an increase in insulin sensitivity. Indeed, muscles of well-trained athletes may contain high concentrations of triacylglycerols and still maintain exquisite sensitivity to insulin. These alterations of muscle biochemistry are some of the molecular manifestations of the training effect of exercise.
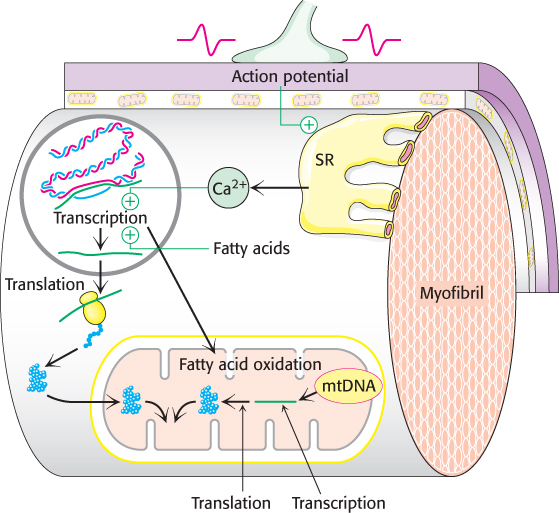
FIGURE 27.10Exercise results in mitochondrial biogenesis and enhanced fat metabolism. An action potential causes Ca2+ release from the sarcoplasmic reticulum (SR), the muscle-cell equivalent of the endoplasmic reticulum. The Ca2+, in addition to instigating muscle contraction, activates nuclear transcription factors that stimulate the expression of specific genes. The products of these genes, in conjunction with the products of mitochondrial genes, are responsible for mitochondrial biogenesis. Fatty acids activate a different set of genes that increase the fatty acid oxidation capability of mitochondria.
[Information from D. A. Hood, J Appl. Physiol. 90:1137–1157, 2001, Fig. 2.]
Fuel choice during exercise is determined by the intensity and duration of activity
In keeping with our theme of energy use under different physiological conditions, we now examine how fuels are used in different types of exercise. The fuels used in anaerobic exercises—sprinting, for example—differ from those used in aerobic exercises—such as distance running. The selection of fuels during these different forms of exercise illustrates many important facets of energy transduction and metabolic integration. ATP directly powers myosin, the protein immediately responsible for converting chemical energy into movement (Chapter 35). However, the amount of ATP in muscle is small. Hence, the power output and, in turn, the velocity of running depend on the rate of ATP production from other fuels. As shown in Table 27.3, creatine phosphate (phosphocreatine) can swiftly transfer its high-potential phosphoryl group to ADP to generate ATP. However, the amount of creatine phosphate, like that of ATP itself, is limited. Creatine phosphate and ATP can power intense muscle contraction for 5 to 6 s. Maximum speed in a sprint can thus be maintained for only 5 to 6 s (Figure 15.7). Thus, the winner in a 100-meter sprint is the runner who both achieves the highest initial velocity and then slows down the least.
TABLE 27.3 Fuel sources for muscle contraction
|
|
Maximal rate of ATP production (mmol s−1) |
Total ~P available (mmol) |
|
|
|
|
|
|
|
|
Conversion of muscle glycogen into lactate |
|
|
Conversion of muscle glycogen into CO2 |
|
|
Conversion of liver glycogen into CO2 |
|
|
Conversion of adipose-tissue fatty acids into CO2 |
|
|
Note: Fuels stored are estimated for a 70-kg person having a muscle mass of 28 kg. Data from E. Hultman and R. C. Harris. In Principles of Exercise Biochemistry, edited by J. R. Poortmans (Karger, 2004), pp. 78–119. |
During a ~10 second sprint, the ATP level in muscle drops from 5.2 to 3.7 mM, and that of creatine phosphate decreases from 9.1 to 2.6 mM. Anaerobic glycolysis provides fuel to make up for the loss of ATP and creatine phosphate. A 100-meter sprint is powered by stored ATP, creatine phosphate, and the anaerobic glycolysis of muscle glycogen. The conversion of muscle glycogen into lactate can generate a good deal more ATP, but the rate is slower than that of phosphoryl-group transfer from creatine phosphate. Because of anaerobic glycolysis, the blood-lactate level is elevated from 1.6 to 8.3 mM. The release of H+ from the intensely active muscle concomitantly lowers the blood pH from 7.42 to 7.24. Sprinting is powered by fast twitch muscle fiber (type IIb) specialized for anaerobic glycolysis. Recall that one of the effects of high-intensity training is to increase the amount of lactate transporters in the membranes of slow twitch fibers (type 1), which remove lactate from the blood and slow the fall in blood pH (Section 16.4). Nonetheless, the pace of a 100-meter sprint cannot be sustained in a 1000-meter run (~132 s) for two reasons. First, creatine phosphate is consumed within a few seconds. Second, the lactate produced would cause acidosis. Thus, alternative fuel sources are needed.
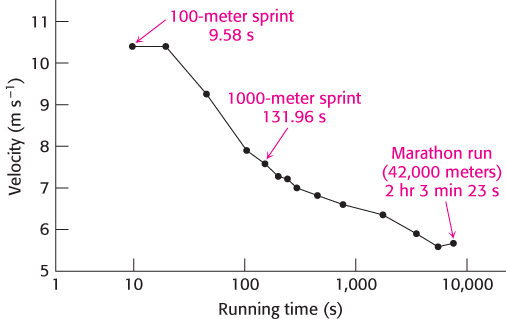
FIGURE 27.11Dependency of the velocity of running on the duration of the race. The values shown are world track records.
Let us examine the fuel use of a middle-distance runner. The complete oxidation of muscle glycogen to CO2 by aerobic respiration substantially increases the energy available to power the 1000-meter run, but this aerobic process is a good deal slower than anaerobic glycolysis. Because ATP is produced more slowly by oxidative phosphorylation than by glycolysis (Table 27.3), the runner’s pace is necessarily slower than in a 100-meter sprint. The championship velocity for the 1000-meter run is about 7.6 m s−1, compared with approximately 10.4 m s−1 for the 100-meter event (Figure 27.11).
The running of a marathon (26 miles 385 yards, or 42,200 meters) requires a different selection of fuels and is characterized by cooperation (depending on the capabilities of the runner) between muscle, liver, and adipose tissue. Liver glycogen complements muscle glycogen as an energy store that can be tapped. Elite marathoners can use the aerobic combustion of glucose to power the entire race, provided they consume fuels during the race. However, for most of us, the total body glycogen stores (103 mol of ATP at best) are insufficient to provide the 150 mol of ATP needed for this grueling event. Much larger quantities of ATP can be obtained by the oxidation of fatty acids derived from the breakdown of fat in adipose tissue, but the maximal rate of ATP generation is slower yet than that of glycogen oxidation and is more than 10-fold slower than that with creatine phosphate. Thus, ATP is generated much more slowly from high-capacity stores than from limited ones, accounting for the different velocities of anaerobic and aerobic events. Fats are rapidly consumed in activities such as distance running, explaining why extended aerobic exercise is beneficial for people who are insulin resistant.
Is it possible to determine the contribution of each fuel as a function of exercise intensity? The percentage contribution of each fuel can be measured with the use of a respirometer, which measures the respiratory quotient (RQ), the ratio of CO2 produced to O2 consumed. Consider the complete combustion of glucose:
The RQ for glucose is 1. Now consider the oxidation of a typical fatty acid, palmitate:
The RQ for palmitate oxidation is 0.7. Thus, as aerobic exercise intensity increases, the RQ will rise from 0.7 (only fats are used as fuel) to 1.0 (only glucose is used as fuel). Between these values, a mixture of fuels is used (Figure 27.12).
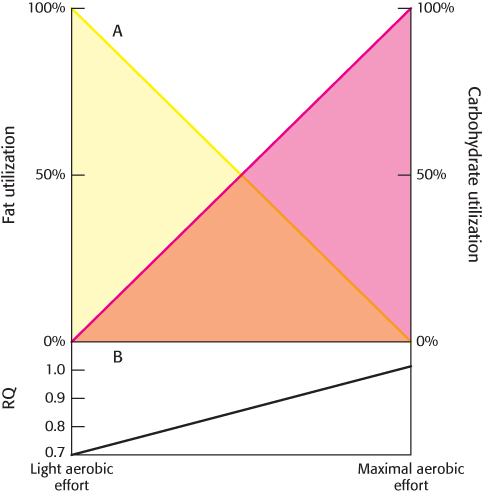
FIGURE 27.12An idealized representation of fuels use as a function of aerobic exercise intensity. (A) With increased exercise intensity, the use of fats as fuels falls as the utilization of glucose increases. (B) The respiratory quotient (RQ) measures the alteration in fuel use.
What is the optimal mix of fuels for use during a marathon? As suggested above, this is a complex question that varies with the athlete and level of training. Studies have shown that, when muscle glycogen has been depleted, the power output of the muscle falls to approximately 50% of maximum. Power output decreases despite the fact that ample supplies of fat are available, suggesting that fats can supply only about 50% of maximal aerobic effort. Indeed, depletion of glycogen stores during a race is referred to as “bonking” or “hitting the wall,” with the result that the athlete must greatly reduce the pace.
How is an optimal mix of these fuels achieved? A low blood-sugar level leads to a high glucagon/insulin ratio, which in turn mobilizes fatty acids from adipose tissue. Fatty acids readily enter muscle, where they are degraded by β oxidation to acetyl CoA and then to CO2. The elevated acetyl CoA level decreases the activity of the pyruvate dehydrogenase complex to block the conversion of pyruvate into acetyl CoA. Hence, fatty acid oxidation decreases the funneling of glucose into the citric acid cycle and oxidative phosphorylation. Glucose is spared so that just enough remains available at the end of the marathon to increase the pace as the finish line draws near. The simultaneous use of both fuels gives a higher mean velocity than would be attained if glycogen were totally consumed before the start of fatty acid oxidation. It is important to bear in mind that fuel utilization is only one of many factors that determine running ability.
If carbohydrate-rich meals are consumed after glycogen depletion, glycogen stores are rapidly restored. In addition, glycogen synthesis continues during the consumption of carbohydrate-rich meals, increasing glycogen stores far above normal. This phenomenon is called “super compensation” or, more commonly, carbo-loading.