36.4The Clinical Development of Drugs Proceeds Through Several Phases
The Clinical Development of Drugs Proceeds Through Several Phases
In the United States, the FDA requires demonstration that drug candidates be effective and safe before they may be used in human beings on a large scale. This requirement is particularly true for drug candidates that are to be taken by people who are relatively healthy. More side effects are acceptable for drug candidates intended to treat significantly ill patients such as those with serious forms of cancer, where there are clear, unfavorable consequences for not having an effective treatment.
1052
Clinical trials are time consuming and expensive
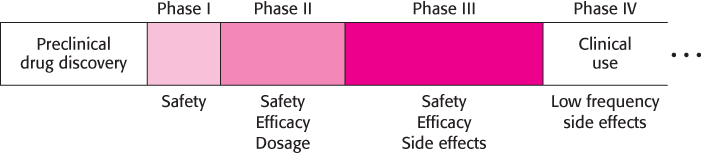
Clinical trials test the effectiveness and potential side effects of a candidate drug before it is approved by the FDA for general use. These trials proceed in at least three phases (Figure 36.27). In Phase I, a small number (typically from 10 to 100) of usually healthy volunteers take the drug for an initial study of safety. These volunteers are given a range of doses and are monitored for signs of toxicity. The efficacy of the drug candidate is not specifically evaluated.
In Phase II, the efficacy of the drug candidate is tested in a small number of persons who might benefit from the drug. Further data regarding the drug’s safety are obtained. Such trials are often controlled and double-
The power of the placebo effect—that is, the tendency to perceive improvement in a subject who believes that he or she is receiving a potentially beneficial treatment—
In Phase III, similar studies are performed on a larger and more diverse population. This phase is intended to more firmly establish the efficacy of the drug candidate and to detect side effects that may develop in a small percentage of the subjects who receive treatment. Thousands of subjects may participate in a typical Phase III study.
Clinical trials can be extremely costly. Hundreds or thousands of patients must be recruited and monitored for the duration of the trial. Many physicians, nurses, clinical pharmacologists, statisticians, and others participate in the design and execution of the trial. Costs can run from tens of millions to hundreds of millions of dollars. Extensive records must be kept, including documentation of any adverse reactions. These data are compiled and submitted to the FDA. The full cost of developing a drug is currently estimated to be more than $800 million.
Even after a drug has been approved and is in use, difficulties can arise. Clinical trials run after a drug has entered the market, referred to as Phase IV studies, are designed to identify low-
1053
The evolution of drug resistance can limit the utility of drugs for infectious agents and cancer
Many drugs are used for long periods of time without any loss of effectiveness. However, in some cases, particularly for the treatment of cancer or infectious diseases, drug treatments that were initially effective become less so. In other words, the disease becomes resistant to the drug therapy. Why does this resistance develop? Infectious diseases and cancer have a common feature—
The HIV protease inhibitors discussed earlier provide an important example of the evolution of drug resistance. Retroviruses are very well suited to this sort of evolution because reverse transcriptase carries out replication without a proofreading mechanism. In a genome of approximately 9750 bases, each possible single point mutation is estimated to appear in a virus particle more than 1000 times per day in each infected person. Many multiple mutations also occur. Most of these mutations either have no effect or are detrimental to the virus. However, a few of the mutant virus particles encode proteases that are less susceptible to inhibition by the drug. In the presence of an HIV protease inhibitor, these virus particles will tend to replicate more effectively than does the population at large. With the passage of time, the less-
Pathogens may become resistant to antibiotics by completely different mechanisms. Some pathogens contain enzymes that inactivate or degrade specific antibiotics. For example, many bacteria are resistant to β-lactams such as penicillin because they contain β-lactamase enzymes. These enzymes hydrolyze the β-lactam ring and render the drugs inactive.
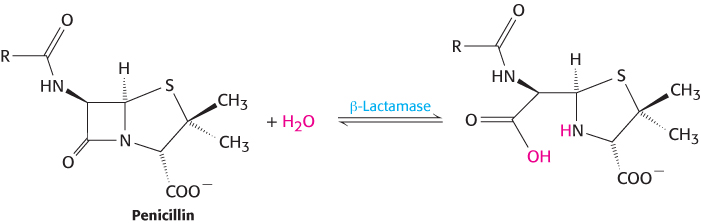
Many of these enzymes are encoded in plasmids, small circular pieces of DNA often carried by bacteria. Many plasmids are readily transferred from one bacterial cell to another, transmitting the capability for antibiotic resistance. Plasmid transfer thus contributes to the spread of antibiotic resistance, a major health-
Drug resistance commonly emerges in the course of cancer treatment. Cancer cells are characterized by their ability to grow rapidly without the constraints that apply to normal cells. Many drugs used for cancer chemotherapy inhibit processes that are necessary for this rapid cell growth. However, individual cancer cells may accumulate genetic changes that mitigate the effects of such drugs. These altered cancer cells will tend to grow more rapidly than others and will become dominant within the cancer-
1054
Cancer patients often take multiple drugs concurrently in the course of chemotherapy and, in many cases, cancer cells become simultaneously resistant to many or all of them. This multiple-